Chapter: Biochemistry: Cell Membrane
Osmosis and Biological Significance
Osmosis
If a protein solution is separated by a
semipermeable membrane from pure water, water tends to flow from the latter to
the former. The property of the movement of solvent particles is called as
osmosis. Osmosis is the net diffusion of water from the dilute solution to the
concentrated solution. Osmosis is a colligative property of solution that
depends on the number of molecules or ions of the solute in the solutions.
Osmol units give the number of osmotically active particles per mole of a
solute. Each mole of a non-ionized solute is equivalent to 1 osmol. Osmolarity
of a solution is its solute concentration in osmols / litre. Osmolality of a
solution is its solute concentrations in osmols/kg of the solvent.
Two solutions with identical osmotic pressures
are called as isoosmotic solutions. A solution having lower or higher osmotic
pressure with respect to the other is called as hypo-osmotic or hyperosmotic
solutions respectively.
The plasma membrane is a semipermeable membrane
and it allows only certain solutes to diffuse. The osmotic pressure exhibited
by these impermeable solutes is called as the tonicity of the solution.
Tonicity is an important physiological parameter.
Two solutions with identical tonicities are
called as isotonic solutions. A solution having lower or higher tonicities with
respect to the other is called as hypotonic or hypertonic solutions
respectively.
Biological Significance
·
Hemolysis
and Crenation. The physiological or isotonic saline is 0.9% NaCl. When red
blood cells are suspended in 0.3% NaCl (hypotonic solution), water will enter
into the cells and the cell will burst releasing all its contents. This kind of
lysis is called as hemolysis. The resulting membranes are called as ghosts. On
the other hand, when the cells are placed in 1.5% NaCl, water comes out of the
cell, leading to shrinkage of cells. The process is called as crenation.
·
The
erythrocyte fragility test is based upon the osmotic diffusion property. The
ability of the membrane to withstand hypotonic solution depends upon the
integrity of the membrane. Certain genetic disorders like sickle cell anemia
and deficiency of vitamin E makes the erythrocyte membrane more fragile.
·
Osmotic
pressure of blood is largely due to its mineral ions such as sodium, potassium,
chloride, calcium and protein. The osmotic pressure exerted by proteins is of
considerable biological significance owing to the impermeability of the plasma
membrane to the colloidal particles.
·
Absorption
of water in the intestine is due to osmosis. Formation of urine in the kidneys
may be attributed to osmotic pressure. The net difference in the hydrostatic
pressure and osmotic pressure is responsible for the filtration of water at the
arterial end of the capillary and the reabsorption of the same at the venous
end. At the arterial end, the hydrostatic pressure is 22 mmHg and the osmotic
pressure is 15 mm Hg. The pressure to drive out the fluid is 7 mm Hg.
·
At
the venous end, the hydrostatic pressure is 15 mm Hg and osmotic pressure is 7
mm Hg. The net absorption pressure to draw water back into the capillaries is
15 – 7 = 8 mm Hg. This is called as Starling's hypothesis.
·
The
renal excretion of water is regulated partly by the osmotic pressure exerted by
the colloids in the blood plasma. Increased urination (polyuria) occurring in
diabetes patients is due to the increased water retention by the urinary
glucose.
·
Donnan Membrane Equilibrium
Let us consider two compartments separated by a semi permeable membrane,
which is permeable to water and crystalloids, but not to colloidal particles.
One of the compartment (A) is filled with a moles of NaCl, and the other
compartment (B) is filled with b moles of NaR, in which R happens to be a non
diffusible ion.
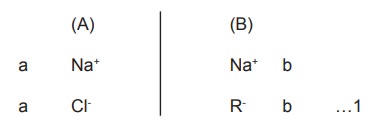
NaCl diffuses from (A) to (B) and after some
time, the system attains equilibrium. At equilibrium, let us consider that x
moles of NaCl have diffused from (A) to (B). So, the ionic concentration at equilibrium in both
the compartments will be as follows,
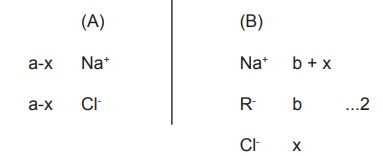
At equilibrium, the number of ions that move
from one compartment to other will be equal, and this will occur only, when the
ionic products of the concerned ions are equal.
Therefore, [Na+][Cl-] in both the compartments
at equilibrium should be equal.
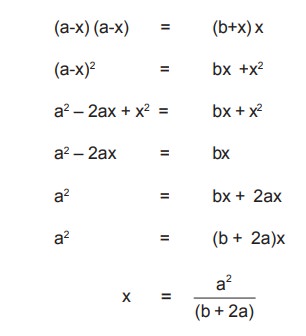
On substituting numerical values for a and b as
2 and 1 moles respectively,
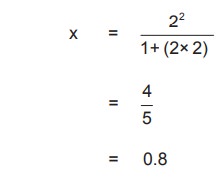
Calculating the total moles present in
compartment (A) and (B) at equilibrium.
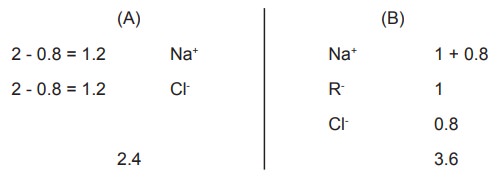
From this we can derive that:
·
The
concentration of solutes in the non-diffusible ion side (B) is greater than the
other.
·
There
will be accumulation of the oppositely charged ion (Na+) in the side
containing the non-diffusible ion (R-).
In biological systems, Donnan membrane
equilibrium prevails due to the non-diffusible proteins and is also significant
for the functional aspects of the cell.
If the non-diffusible ion happens to be R- and
one of the diffusible ion H+, then there will be a change in the pH. Due to
imbalance in the electrolytes, swelling of proteins occur, which is called as
Donnan osmotic effect.
Related Topics