Chapter: Biochemistry: Cell Membrane
Buffers
Buffers
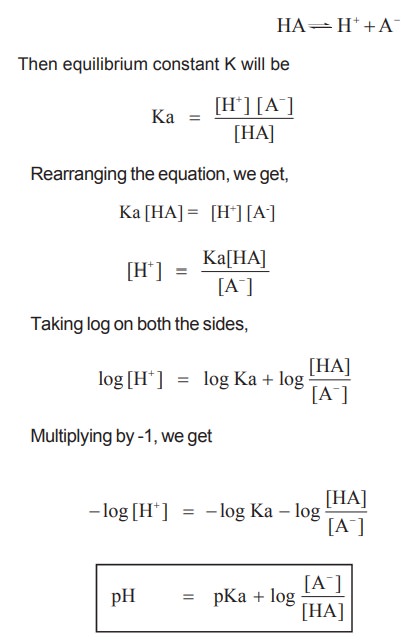
A buffer may be defined as a solution which
resists the change in pH that will occur on addition of small quantities of
acid or base to the solution. Buffers are mixtures of weak acid and its salt or
weak base and its salt. The pH of the solution is defined as the negative
logarithm of hydrogen ion concentration. The pH of buffers are determined by
Henderson Haselbach equation, which is derived as follows
Let us consider a weak acid that ionizes as
follows
The pH of blood is 7.4 and it should be
maintained constant . If pH increases above 7.5, alkalosis occurs and beyond
7.8 death occurs.
If it falls below, 7.3, acidosis occurs and
below 7.0 is incompatible for life. Due to metabolism and dietary intake, large
quantities of acids and bases are produced in the body and they have to be
transported through blood for elimination. This should occur without any major
changes in the pH. This is effectively done in the body by means of the buffers
present in the blood and by two mechanisms, namely the respiratory mechanism
and the renal mechanism.
The buffer systems of blood are as follows
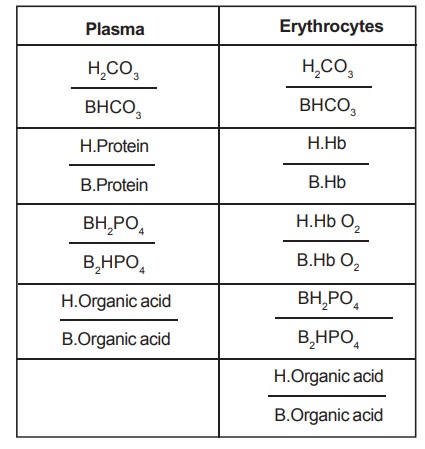
The numerators are acid components and the denominators
are salts.
Since the concentrations of phosphate and
organic acids are low in plasma, they do not play a major role in regulation of
pH.
The major buffer in plasma is bicarbonate buffer
and the pKa of carbonic acid is 6.1. Substituting it in the Henderson
Hasselbach equation,
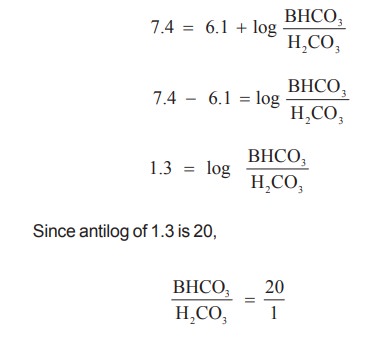
To effectively maintain the pH of blood,
according to Henderson Hasselbach equation, the ratio of bicarbonate to
carbonic acid should be 20 : 1. The carbon dioxide produced by metabolism is
buffered by the hemoglobin buffer system as follows.
Hemoglobin buffer system
The buffering capacity of hemoglobin is due to
the presence of imidazole groups in its histidine residues. The degree of
dissociation of the imidazole groups is dependant upon the degree of oxygenation
of Hb. If hemoglobin is oxygenated, it is more acidic and therefore exists in
its dissociated form. When it is not bound with oxygen, it will be in the
reduced form.
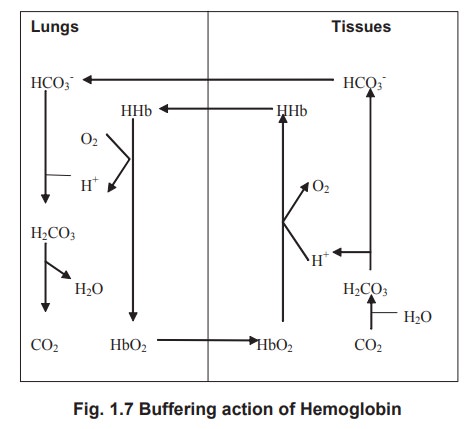
In the tissues, where oxygen tension is reduced,
HbO2 dissociates to give oxygen to the tissues. In turn, the CO2
produced in the tissues will combine with H2O to form H2CO3,
which dissociates to H+ and HCO3-. The reduced
Hb devoid of O2 combines with H+ ions to form HHb resulting a very
little change in the pH.
When the blood returns to the lungs, O2
tension in the lungs is high resulting in the oxygenation of Hb. As mentioned
earlier, HbO2 has lesser affinity to H+ and releases it. It combines
with HCO3- ions to form H2CO3 that
dissociates to H2O and CO2.
It has been found that more than 80% of the
buffering capacity of blood is due to red blood cells. But the buffered HCO3-
is transported in the plasma. The process of transport of the formed HCO3-
from the RBCs into the plasma needs chloride ions and the phenomenon is called
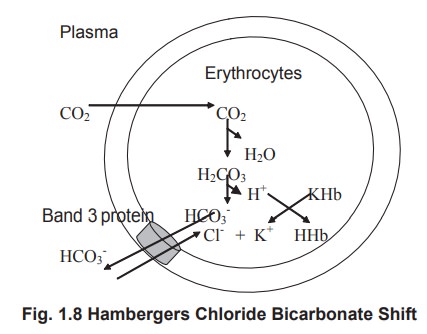
as Hamberger’s chloride bicarbonate shift.
When CO2 liberated from the tissues
enters theRBC via plasma, it combines with water to form carbonic acid, the
reaction catalysed by an enzyme called as carbonic anhydrase. The same enzyme
can also dissociate carbonic acid to carbon dioxide and water. Carbonic acid
dissociates into HCO3- and H+ ions.
The formed bicarbonate is exchanged for one
chloride ion with the plasma. The chloride that enters the cell forms neutral
potassium chloride in the cell. The bicarbonate that enters the plasma reacts
with the sodium ions to form sodium bicarbonate. Thus, the bicarbonate ions are
transported in the plasma.
Regulation by Respiratory mechanism
Respiratory mechanism plays an important role in
the regulation of acid-base balance because the respiratory centre is sensitive
to the changes in pCO2 .
If there is an increase in pCO2,
increased respiration occurs, helping to remove the excess CO2. This
continues until the blood regains normal pCO2 and pH. Similarly a
fall in the pCO2 leads to slow, shallow respiration, hypoventilation
and retention of CO2.
Regulation by renal mechanism
The lungs can remove only volatile acids like CO2
but not the organic acids like lactic acid and pyruvic acids. These acids are
effectively buffered by the bicarbonate system, but at the expense of the
bicarbonate, which is called as the alkali reserve of the body. Lungs can
eliminate H2CO3, but cannot restore bicarbonate. This is
doneby the kidneys, which are the ultimate regulators of acid base balance. In
acidemia, inorder to bring the low pH to normal, the excessive H+ ions should
be excreted and bicarbonate excretion should be reduced. This is done by
excreting a highly acidic urine (pH 4.5). On the other hand, during alkalemia,
the kidneys excrete the excess bicarbonate producing an alkaline urine (pH
8.2). The three important mechanisms attributed by the kidneys to regulate the
blood pH are
·
Reabsorption
of bicarbonate
·
Buffering
by phosphate buffers
·
Formation
of ammonium ions.
Related Topics