Chapter: Medical Physiology: Membrane Physiology, Nerve, and Muscle : Transport of Substances Through the Cell Membrane
Osmosis Across Selectively Permeable Membranes - ÔÇťNet DiffusionÔÇŁ of Water
Osmosis Across Selectively Permeable Membranes - ÔÇťNet DiffusionÔÇŁ of Water
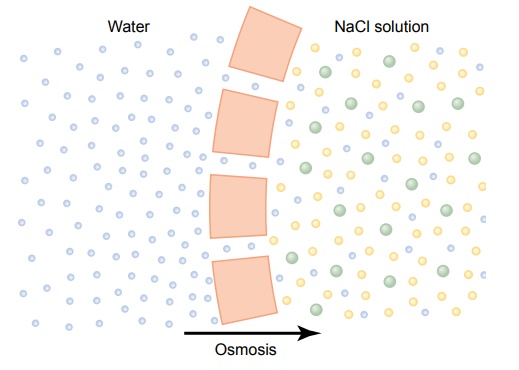
By far the most abundant substance that diffuses through the cell membrane is water. Enough water ordinarily diffuses in each direction through the red cell membrane per second to equal about 100 times thevolume of the cell itself. Yet, normally, the amount thatdiffuses in the two directions is balanced so precisely that zero net movement of water occurs. Therefore, the volume of the cell remains constant. However, under certain conditions, a concentration difference for water can develop across a membrane, just as concentration differences for other substances can occur. When this happens, net movement of water does occur across the cell membrane, causing the cell either to swell or to shrink, depending on the direction of the water move-ment. This process of net movement of water caused by a concentration difference of water is called osmosis.
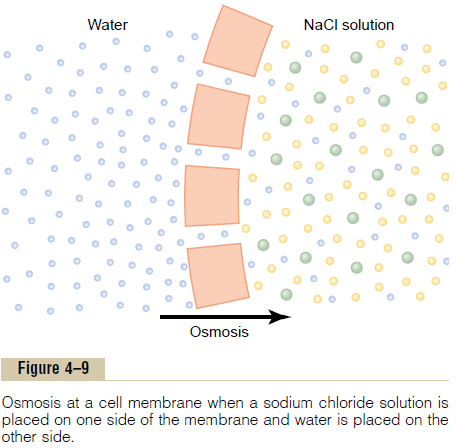
To give an example of osmosis, let us assume the conditions shown in Figure 4ÔÇô9, with pure water on one side of the cell membrane and a solution of sodium chloride on the other side. Water molecules pass through the cell membrane with ease, whereas sodium and chloride ions pass through only with diffi-culty. Therefore, sodium chloride solution is actually a mixture of permeant water molecules and nonperme-ant sodium and chloride ions, and the membrane is said to be selectively permeable to water but much less so to sodium and chloride ions. Yet the presence of the sodium and chloride has displaced some of the water molecules on the side of the membrane where these ions are present and, therefore, has reduced the con-centration of water molecules to less than that of pure water. As a result, in the example of Figure 4ÔÇô9, more water molecules strike the channels on the left side, where there is pure water, than on the right side, where the water concentration has been reduced. Thus, net movement of water occurs from left to rightÔÇöthat is, osmosis occurs from the pure water into the sodiumchloride solution.
Osmotic Pressure
If in Figure 4ÔÇô9 pressure were applied to the sodium chloride solution, osmosis of water into this solution would be slowed, stopped, or even reversed. The exact amount of pressure required to stop osmosis is called the osmotic pressure of the sodium chloride solution.
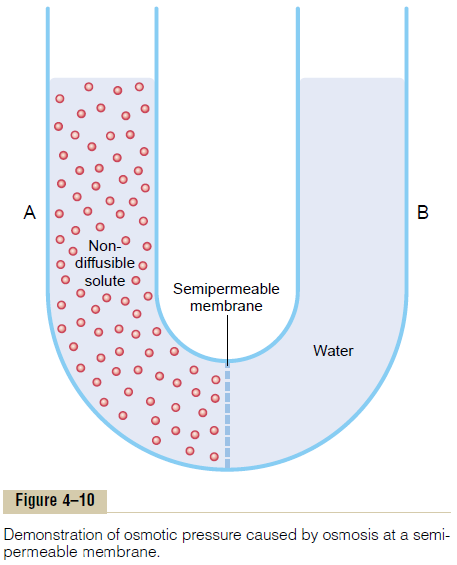
The principle of a pressure difference opposing osmosis is demonstrated in Figure 4ÔÇô10, which shows a selectively permeable membrane separating two columns of fluid, one containing pure water and the other containing a solution of water and any solute that will not penetrate the membrane. Osmosis of water from chamber B into chamber A causes the levels of the fluid columns to become farther and farther apart, until eventually a pressure difference develops between the two sides of the membrane great enough to oppose the osmotic effect. The pressure dif-ference across the membrane at this point is equal to the osmotic pressure of the solution that contains the nondiffusible solute.
Importance of Number of Osmotic Particles (Molar Concentra-tion) in Determining Osmotic Pressure. The osmotic pres-sure exerted by particles in a solution, whether they are molecules or ions, is determined by the number of particles per unit volume of fluid, not by the mass of the particles. The reason for this is that each particle in a solution, regardless of its mass, exerts, on average, the same amount of pressure against the membrane. That is, large particles, which have greater mass (m) than small particles, move at slower velocities (v). The small particles move at higher velocities in such a way that their average kinetic energies (k), determined by the equation
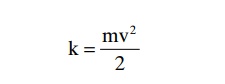
are the same for each small particle as for each large particle. Consequently, the factor that determines the osmotic pressure of a solution is the concentration of the solution in terms of number of particles (which is the same as its molar concentration if it is a nondisso-ciated molecule), not in terms of mass of the solute.
ÔÇťOsmolalityÔÇŁÔÇöThe Osmole. To express the concentrationof a solution in terms of numbers of particles, the unit called the osmole is used in place of grams.
One osmole is 1 gram molecular weight of osmoti-cally active solute. Thus, 180 grams of glucose, which is 1 gram molecular weight of glucose, is equal to 1 osmole of glucose because glucose does not dissociate into ions. Conversely, if a solute dissociates into two ions, 1 gram molecular weight of the solute will become 2 osmoles because the number of osmotically active particles is now twice as great as is the case for the nondissociated solute. Therefore, when fully disso-ciated, 1 gram molecular weight of sodium chloride, 58.5 grams, is equal to 2 osmoles.
Thus, a solution that has 1 osmole of solute dissolvedin each kilogram of water is said to have an osmolality of 1 osmole per kilogram,and a solution that has1/1000 osmole dissolved per kilogram has an osmo-lality of 1 milliosmole per kilogram. The normal osmolality of the extracellular and intracellular fluids is about 300 milliosmoles per kilogram of water.
Relation of Osmolality to Osmotic Pressure. At normal bodytemperature, 37┬░C, a concentration of 1 osmole per liter will cause 19,300 mm Hg osmotic pressure in the solution. Likewise, 1 milliosmole per liter concentra-tion is equivalent to 19.3 mm Hg osmotic pressure. Multiplying this value by the 300 milliosmolar con-centration of the body fluids gives a total calculated osmotic pressure of the body fluids of 5790 mm Hg. The measured value for this, however, averages only about 5500 mm Hg. The reason for this difference is that many of the ions in the body fluids, such as sodium and chloride ions, are highly attracted to one another; consequently, they cannot move entirely unrestrained in the fluids and create their full osmotic pressure potential. Therefore, on average, the actual osmotic pressure of the body fluids is about 0.93 times the cal-culated value.
The Term ÔÇťOsmolarity.ÔÇŁ Because of the difficulty of meas-uring kilograms of water in a solution, which is required to determine osmolality, osmolarity, which is the osmolar concentration expressed as osmoles per liter ofsolution rather than osmoles per kilogram of water, is used instead. Although, strictly speaking, it is osmoles per kilogram of water (osmolality) that determines osmotic pressure, for dilute solutions such as those in the body, the quantitative differences between osmo-larity and osmolality are less than 1 per cent. Because it is far more practical to measure osmolarity than osmo-lality, this is the usual practice in almost all physiologic studies.
Related Topics