Chapter: Medical Physiology: Membrane Physiology, Nerve, and Muscle : Transport of Substances Through the Cell Membrane
Factors That Affect Net Rate of Diffusion
Factors That Affect Net Rate of Diffusion
By now it is evident that many substances can diffuse through the cell membrane. What is usually important is the net rate of diffusion of a substance in the desired direction. This net rate is determined by several factors.
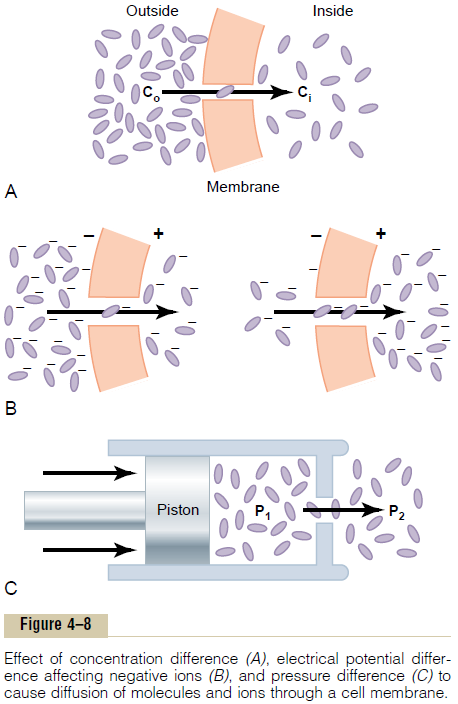
Effect of Concentration Difference on Net Diffusion Through a Membrane. Figure 4–8Ashows a cell membrane with asubstance in high concentration on the outside and low concentration on the inside. The rate at which the sub-stance diffuses inward is proportional to the con-centration of molecules on the outside, because this concentration determines how many molecules strike the outside of the membrane each second. Conversely, the rate at which molecules diffuse outward is propor-tional to their concentration inside the membrane. Therefore, the rate of net diffusion into the cell is pro-portional to the concentration on the outside minus the concentration on the inside, or:
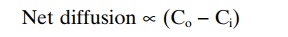
in which Co is concentration outside and Ci is concen-tration inside.
Effect of Membrane Electrical Potential on Diffusion of Ions— The “Nernst Potential.” If an electrical potential isapplied across the membrane, as shown in Figure 4–8B, the electrical charges of the ions cause them to move through the membrane even though no concen-tration difference exists to cause movement. Thus, in the left panel of Figure 4–8B, the concentration of negative ions is the same on both sides of the mem-brane, but a positive charge has been applied to the right side of the membrane and a negative charge to the left, creating an electrical gradient across the mem-brane. The positive charge attracts the negative ions, whereas the negative charge repels them. Therefore, net diffusion occurs from left to right.
After much time, large quantities of negative ions have moved to the right, creating the condition shown in the right panel of Figure 4–8B, in which a concentration difference of the ions has developed in the direction opposite to the electrical potential difference. The concentration dif-ference now tends to move the ions to the left, while the electrical difference tends to move them to the right. When the concentration difference rises high enough, the two effects balance each other. At normal body temperature (37°C), the electrical difference that will balance a given concentration difference ofunivalent ions—such as sodium (Na+) ions—can bedetermined from the following formula, called the
Nernst equation:
EMF (in millivolts) = ± 61 log C1/ C2
in which EMF is the electromotive force (voltage) between side 1 and side 2 of the membrane, C1 is the concentration on side 1, and C2 is the concentration on side 2. This equation is extremely important in under-standing the transmission of nerve impulses.
Effect of a Pressure Difference Across the Membrane. Attimes, considerable pressure difference develops between the two sides of a diffusible membrane. This occurs, for instance, at the blood capillary mem-brane in all tissues of the body. The pressure is about 20 mm Hg greater inside the capillary than outside.
Pressure actually means the sum of all the forces of the different molecules striking a unit surface area at a given instant. Therefore, when the pressure is higher on one side of a membrane than on the other, this means that the sum of all the forces of the molecules striking the channels on that side of the membrane is greater than on the other side. In most instances, this is caused by greater numbers of molecules striking the membrane per second on one side than on the other side. The result is that increased amounts of energy are available to cause net movement of molecules from the high-pressure side toward the low-pressure side. This effect is demonstrated in Figure 4–8C, which shows a piston developing high pressure on one side of a “pore,” thereby causing more molecules to strike the pore on this side and, therefore, more molecules to “diffuse” to the other side.
Related Topics