Chapter: Pharmaceutical Drug Analysis: Nuclear Magnetic Resonance Spectroscopy
Nuclear Magnetic Resonance Spectroscopy
NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
INTRODUCTION
Nuclear Magnetic Resonance (NMR) spectroscopy just like
IR and UV is regarded as a process whereby
energy from an external source is absorbed and brings about a change or
resonance to an ‘excited’ or high energy state. The energy required for NMR
lies in the low energy or long wavelength radio-frequency end of the
electromagnetic spectrum.
Consequent to the magnetic properties of nuclei arising
from the axial spin, the emerging radio-frequency gets absorbed in a magnetic
field. Therefore, for a particular nucleus an NMR absorption spectrum
invariably comprises one to several groups of absorption lines in the
ratio-frequency portion of the electromagnetic spectrum. Evidently, the
location of peaks indicate the chemical nature of the nucleus, whereas the
multiplets provide information regarding the spatial positions of the
neighbouring nuclei. Hence, NMR is also known as Nuclear Spin Resonance (NSR) spectroscopy.
NMR has accomplished a growth in a geometrical
progression since the early sixties and virtually developed into extremely
potential analytical tools not only useful for elucidation of complex
structural determinations but also equally beneficial in the assay of
pharmaceutical substances.
In reality, NMR spectroscopy has broadened the scope and
absolute possibility for performing more extensive as well as intensive studies
with regard to recording the spectrum of isolated and synthesized organic
molecules in addition to their mechanistic and stereochemical details hitherto
inaccessible. There-fore, NMR spectroscopy finds its applications for compound
identification, by means of a ‘fingerprint
technique’ very much identical to
that used in IR-spectroscopy. Besides, it is invariably utilized as a specific method of assay for the individual
constituents of a mixture.
1. THE NMR PHENOMENON
Following are the five
different aspects that essentially govern the NMR phenomenon, namely :
A. The Spinning Nucleus :
The nucleus of the hydrogen
atom, i.e., the proton, just behaves as if it is a small spinning bar magnet. It does so because it evidently
possesses an electrical charge as well as a mechanical spin. Consequently, a
spinning charged body will generate a magnetic field, and hence the nu-cleus of
hydrogen atom is not an exception.
B. The effect of an External Magnetic Field :
As a ‘compass
needle’ possesses an inherent ten-dency to align itself with the earth’s
magnetic field, the proton not only responds to the influence of an external
magnetic field but also tends to align itself with that field. However, because
of restrictions as applicable to nuclei (not to compass needles) the proton can
only adopt the following two orientations with regard to an external magnetic
field. At this juncture two
situations normally arise, namely :
(a) when proton
is aligned with the field (i.e., at
lower energy state), and
(b) when proton
is opposed to the field (i.e., at
higher energy state).
Some NMR-analysts describe these proton orientations as ‘parallel’ with or ‘antiparallel’ with the applied field.
C. The Precessional Motion :
The proton appears to be
behaving as ‘spinning magnet’ and
there-fore, not only can it align itself with or oppose an external field, but
also may move in a characteristic manner under the influence of the external
magnet.
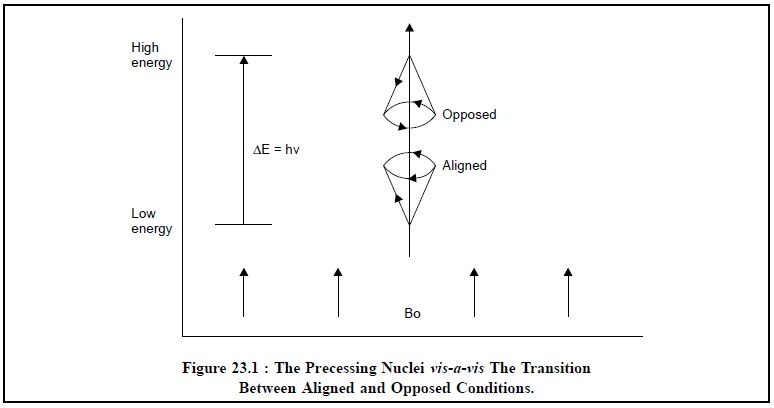
Figure 23.1, represents the precessing nuclei vis-a-vis the transition energy (∆E)
of reorientation of magnetic dipole between the aligned and opposed conditions
when subjected to an external magnetic field (Bo). It is absolutely clear from
this Figure that the proton gets aligned with the external magnetic field only
at a lower energy states, while it becomes opposed to the field at higher
energy states.
However, the energy of the reorientation of magnetic
dipole, ∆E, may be expressed as follows :
∆E = hν
where, h = Planck’s constant, and
v= Frequency of radiation.
In order to understand the precessional motion more
vividly, let us take the example of a spinning ‘top’ and its spinning motion.
The top will (unless absolutely vertical) also perform a comparatively slower waltz-like motion whereby the spinning
axis of the top moves slowly around the vertical. This particular phenomenon is known as the precessional motion and hence, the
‘top’ is generally said to be precessing around the vertical axis of the
earth’s gravitational field. In other words, the precession comes into effect
due to the interaction of spin (i.e.,
gyroscopic motion) with the earth’s
gravity vertically downwards. Therefore, a spinning top will precess, whereas a
static top will fall over (not precess).
D. The Precessional Frequency :
The
spinning frequency of the nucleus does not change at all, whereas the speed of precession does. Therefore, ν ∝ Bo, i.e., the precessional frequency is directly propor-tional to the
strength of the external field Bo.
It designates one of the most important relationships in
NMR-spectroscopy.
Example : A proton expressed to an
external magnetic force of 1.4 T (≡ 14, 000 gauss) will precess ~ 60
million times per second so that the precessional frequency ν =
60 MHz ; and for an external field of 2.3 T, ν ≈
100 MHz, and at 5.1 T, ν ≈ 200 MHz.
E. The Energy Transitions :
Whenever
a proton is precessing in the aligned orientation (low energy) it can absorb energy and pass into the
orientation (high energy) ; and subsequently it can lose this extra energy and
relax back into the aligned state.
Interestingly, the precessing proton can only absorb
energy from the radio frequency source if the precessing frequency is exactly
the same as that of the radio frequency beam ; and when this particular
situation arises, the nucleus and the radio frequency beam are said to be in
resonance, thereby justifying the term ‘nuclear magnetic resonance’.
In NMR spectroscopy, the precessing protons of an organic
molecule, after being duly exposed to a powerful external magnetic field
(ranging between 60-400 MHz), are irradiated with radio frequency energy of the
appropriate frequencies, thereby promoting protons from the low-energy (aligned
state) to the high-energy (opposed state). The absorption of energy is
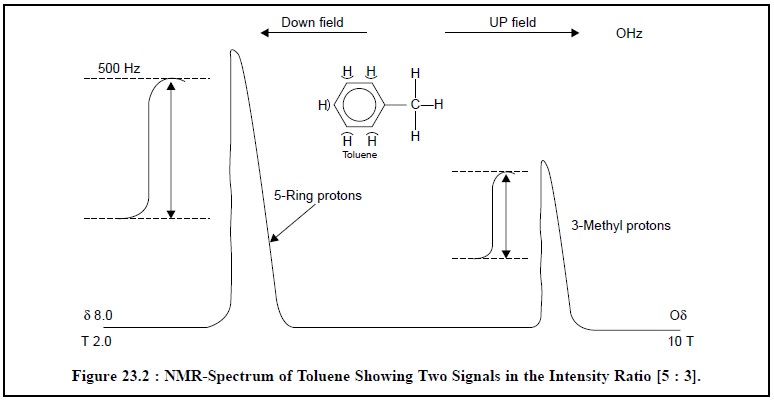
ultimately recorded in the form of NMR spectrum as shown in Figure 23.2.
2. INFORMATIONS PROVIDED BY 1H-NMR (PROTON-NMR)
1H-NMR provides a number of
valuable informations stated below, which are employed for the struc-tural
elucidation as well as assay of important pharmaceutical substances, namely :
(i) To record
differences in the magnetic properties of the various nuclei present,
(ii) To deduce
in large measure the exact locations of these nuclei within the molecule,
(iii) To deduce
how many different types of hydrogen environments are present in the molecule,
(iv) To deduce
which hydrogen atoms are present on neighbouring carbon atoms, and
(v) To measure exactly
how many H-atoms are actually present in each of these environments.
Example : Figure 23.2 depicts the
NMR-spectrum of toluene (C6H5—CH3), which
essentially pos-sesses two different
species of H-atoms, for instance :
(a) methyl
hydrogen atoms (—CH3), and
(b) aromatic ring hydrogen atoms (—C6H5).
Hence, two signals will show-up in the NMR-spectrum
corresponding to these two different chemical and magnetic environments.
Furthermore, the areas under each signal are in the ratio of the number of
protons in each part of the molecule, and thus actual measurement will reveal
that the ratio of these areas is 5 : 3.
Related Topics