Chapter: Pharmaceutical Drug Analysis: Nuclear Magnetic Resonance Spectroscopy
Interpretation of a NMR-Spectrum
INTERPRETATION OF A NMR-SPECTRUM
The interpretation of a NMR-spectrum can be accomplished
by determining the following parameters for each signal methodically as
described below :
1. CHEMICAL SHIFT (δ) (RELATIVE TO REFERENCE
COMPOUND, USUALLY Me4Si)
The chemical shift indicates the environment of the
proton. One may refer to the tables and charts in various reference books* for
approximate ranges of δ for 1H in
different environments.
2. RELATIVE PEAK AREA
This is equal to the height of step of the integration
trace.
In fact, peak area is proportional to the number of
protons causing the signal. Always look for simple ratios e.g., 3 : 1, rather than (say) 14 : 4. A strong singlet (or upfield
triplet) may indicate CH3 ; the corre-sponding integration steps
provide a good starting point for ascertaining the relative number of protons
present in the molecule under investigation.
3. MULTIPLICITY OF THE SIGNAL
The number of peaks in a regularly split signal (e.g., a regularly spaced triplet,
quartet etc., ) or other recognisable splittings (e.g., doublet of doublets etc.,), should be noted carefully.
Therefore, multiplicity and the relative peak heights in
a multiplet provide an useful additional check on the relative number of
protons obtained from the integration of peak areas.
Thus, coupling 1Ha to another 1Hb
may give rise to a doublet or a triplet or a doublet-of-doublet as shown below
:
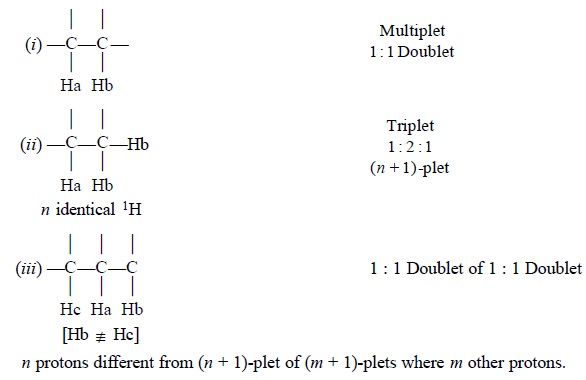
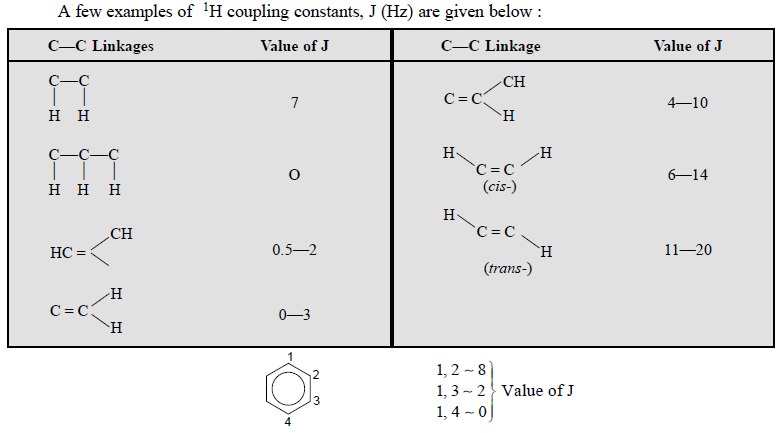
4. COUPLING CONSTANT (J)
It represents regular multiplets. Actually, J is the
separation (in Hertz ; Hz = sec–1) between the peaks of regular
multiplets.
The coupling constants help in the identification of the
coupled nuclei because Jab = Jba : and are therefore, useful in characterizing
the relative orientations of interacting protons.
Related Topics