Chapter: Pharmaceutical Drug Analysis: Nuclear Magnetic Resonance Spectroscopy
Applications of NMR-Spectroscopy in Pharmaceutical Analysis
APPLICATIONS OF NMR-SPECTROSCOPY IN PHARMACEUTICAL ANALYSIS
NMR-spectroscopy has been extensively employed for the
identification testing as well as quantita-tive analysis of pharmaceutical substances.
These two aspects shall be discussed
in the sections that follow :
1. IDENTIFICATION TESTING
The versatility and ability of NMR to distinctly
differentiate nuclei in various intramolecular environ-ments has placed it as
the most reliable and dependable technique for carrying out the identification
testing of a host of pure drugs. Hence, any apparent deviations of the spectrum
of a sample under investigation vis-a-vis
the spectrum of the pure and the authentic pharmaceutical substance usually
give rise to an enormous information
not only confined to the true identity of the substance but also the probable
nature of the impu-rities it possesses.
The survey of literature provides ample evidence of the
NMR spectra of a good number of medicinal compounds belonging to various
categories, namely : sulphonamides ; barbiturates* ; amphetamines** ; steroids
; antihistamines*** ; penicillins and cephalosporins**** to name a few.
2. ASSAY OF DRUGS
A plethora of pure drugs, their respective combinations
and their dosage forms have been assayed by NMR-spectroscopy quantitatively by
various researchers and the result(s) thus obtained were duly verified and
compared with the standard methods prescribed in various official compendia. A few typical examples of such drugs shall be
described briefly here :
A.
Quinidine in Mixtures and
Hydroquinidine*****
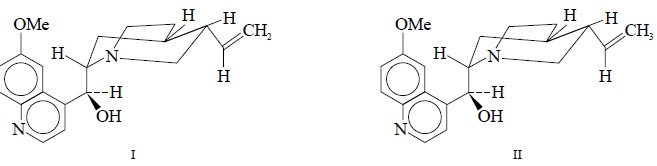
A given sample containing a mixture of quinidine (I) and
hydroquinidine (II) is dissolved in requisite quantity of deutrochloroform
(CDCl3) along with 2, 3, 5-triboromothiophene as the internal standard. The quantitative
determination is carried out by comparing the peak area attributed by ethylene
of (I) at 5.16 ppm to the internal standard peak at 6.93 ppm. The coefficient
of variation was found to be 1%.
B.
Assay Methsuximide and
Phensuximide Capsules*
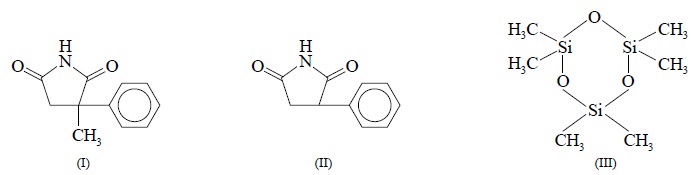
The analysis of methsuximide (I) is performed in carbon
tetrachloride and of phensuximide (II) in 10% v/v dichloromethane in carbon
tetrachloride. In this particular analysis hexamethylcyclotrisiloxane (III) is
employed as an internal standard for
(I) and (II) ; whereas the frequencies are referenced to usual
tetra-methylsilane (TNS).
C.
Assay of Trimethoprim and
Sulphamethoxazole in Tablets and Powders**
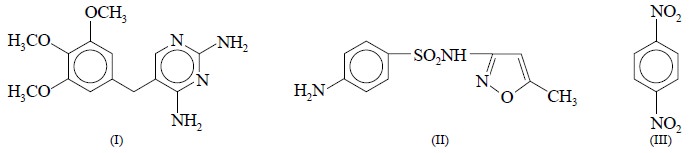
The simultaneous assay of trimethoprim (I) and
sulfamethoxazole (II) present either in tablets or powder may be done
effectively by NMR method.
Here, a powdered sample comprising 1 mg of (1), 50 mg of
(II), and 30 mg of pure 1,4-dinitrobenzene (III) as internal standard is carefully dissolved by heating in 1 ml of
dimethylsulphoxide-d6 and
subse-quently centrifuged to eliminate solid residues, if any. For trimethoprim
(I) : the assay is solely based on the singlets at 3.40 and 3.55 ppm on account
of the aromatic and methoxy protons of (I) respectively. For sulfamethoxazole
(II) the singlet at 2.3 ppm is particularly due to the methyl group of (II) ;
and the singlet at 8 ppm is due to (III). It is, however, pertinent to mention
here that the assay results were fairly in agreement with British
Pharmacopoeial method of analysis. Finally, the NMR-spectroscopic method
coefficient of vari-ation was found to be only 0.9%.
D. Assay of Meprobamate and Mebutamate
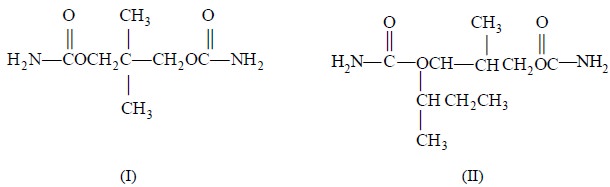
The assay of meprobamate (1) and mebutamate (II) have
been accomplished* by using malonic acid as the internal standard and acetone as the solvent. The results obtained
were fairly comparable to the lengthy official procedures.
E. Assay of Meclizine and Methaqualone
NMR-assay of meclizine (I) and methaqualone (II), besides
a number of other potent hypnotics and their corresponding mixtures have been
successfully carried out using an external standardization procedure
reported**. It is, however, interesting to observe that additional sources of
variability are usually incorpo-rated into an assay employing external
standardization, and the same has been duly shown in the results thus obtained i.e., a large coefficient of variation
to the extent of 4% achieved.
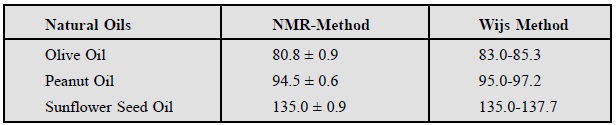
F. Assay of Iodine Values of Natural Oils
Natural oils like : olive, peanut, sunflower seed
contain mostly the triglycerides, which usually give rise to four
characteristic sets of signals in their corresponding PMR-spectra due to the resonance
of alkenyl protons, namely :
(i) 4, C-1
glyceride methylene protons,
(ii) 1, C-2
glyceride methylene proton,
(iii) Methylene
protons directly linked to a double-bond, and
(iv) Remaining
protons on saturated carbon atoms.
Hence, it is possible to measure accurately the
integration curve given out by the combined C-1, and C-2 glyceride methylene
protons that occurs almost separately at 4. Now, employing these as an internal
calibration one may determine conveniently the following two vital informations, such as :
(a) the total
number of alkenyl protons, which is a measure of degree of unsaturation, and,
(b) the total
number of protons, which is a measure of the average molecular weight.
Thus, the iodine values assayed (calculated) from the alkenyl
proton integration*** and the corre-sponding molecular weight match quite
favourably with the results obtained by Wijs Method as shown below :
In addition to the above cited typical examples there are
a quite a few other drug substances which have been duly assayed by
NMR-spectroscopy, thus suggesting the versatility of this technique as an
impor-tant analytical tool.
Related Topics