Chapter: Basic & Clinical Pharmacology : Agents Used in Dyslipidemia
Normal Lipoprotein Metabolism
PATHOPHYSIOLOGY OF HYPERLIPOPROTEINEMIA
NORMAL LIPOPROTEIN METABOLISM
Structure
Lipoproteins
have hydrophobic core regions containing cholesteryl esters and triglycerides
surrounded by unesterified cholesterol, phos-pholipids, and apoproteins.
Certain lipoproteins contain very high-molecular-weight B proteins that exist
in two forms: B-48, formed in the
intestine and found in chylomicrons and their remnants; and B-100, synthesized in liver and found
in VLDL, VLDL remnants (IDL), LDL (formed
from VLDL), and Lp(a) lipoproteins. HDLconsist
of at least 20 discrete molecular species. All species contain apolipoprotein
A-I (apo A-I). Fifty-three other proteins are known to be distributed variously
among the HDL species.
Synthesis & Catabolism
A. Chylomicrons
Chylomicrons are
formed in the intestine and carry triglycerides
of dietary origin, unesterified
cholesterol, and cholesterylesters. They
transit the thoracic duct to the bloodstream.
ACRONYMS
Apo Apolipoprotein
CETP Cholesteryl
ester transfer protein
CK Creatine kinase
HDL High-density
lipoproteins
HMG-CoA 3-Hydroxy-3-methylglutaryl-coenzyme
A
IDL Intermediate-density
lipoproteins
LCAT Lecithin:cholesterol
acyltransferase
LDL Low-density
lipoproteins
Lp(a) Lipoprotein(a)
LPL Lipoprotein
lipase
PPAR Peroxisome
proliferator-activated receptor
VLDL Very-low-density
lipoproteins
Triglycerides are
removed in extrahepatic tissues through a pathway shared with VLDL that
involves hydrolysis by the lipoprotein
lipase (LPL) system. Decrease in particle diameteroccurs as triglycerides
are depleted. Surface lipids and small apo-proteins are transferred to HDL. The
resultant chylomicron remnants are taken up by receptor-mediated endocytosis
into hepatocytes.
B. Very-Low-Density Lipoproteins
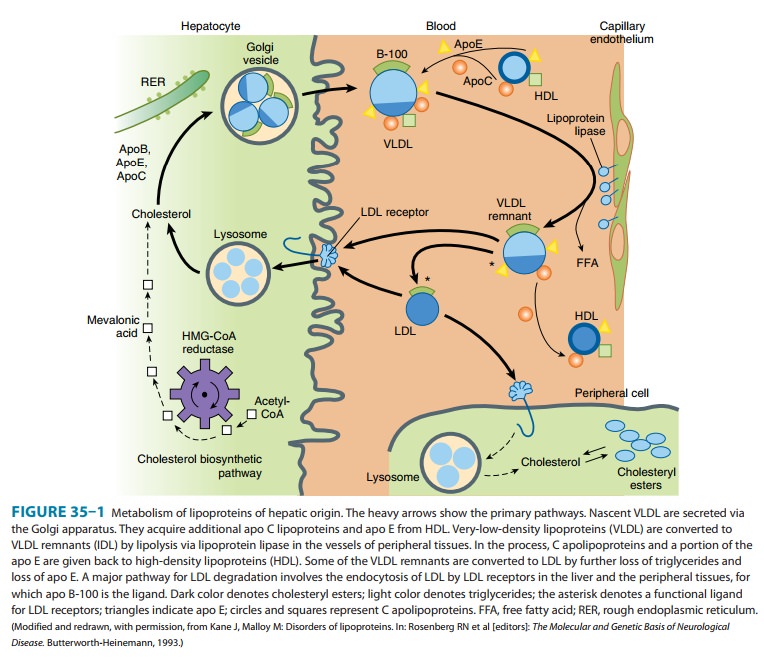
VLDL are secreted by
liver and export triglycerides to peripheral tissues (Figure 35–1). VLDL
triglycerides are hydrolyzed by LPL, yielding free fatty acids for storage in adipose
tissue and for oxida-tion in tissues such as cardiac and skeletal muscle.
Depletion of triglycerides produces remnants (IDL), some of which undergo
endocytosis directly by liver. The remainder is converted to LDL by further
removal of triglycerides mediated by hepatic lipase. This process explains the
“beta shift” phenomenon, the increase of LDL (beta-lipoprotein) in serum as
hypertriglyceridemia subsides. Increased levels of LDL can also result from
increased secretion of VLDL and from decreased LDL catabolism.
C. Low-Density Lipoproteins
LDL is catabolized
chiefly in hepatocytes and other cells by receptor-mediated endocytosis.
Cholesteryl esters from LDL are hydrolyzed, yielding free cholesterol for the
synthesis of cell membranes. Cells also obtain cholesterol by synthesis via a
path-way involving the formation of mevalonic acid by HMG-CoA reductase.
Production of this enzyme and of LDL receptors is transcriptionally regulated
by the content of cholesterol in the cell. Normally, about 70% of LDL is
removed from plasma by hepatocytes. Even more cholesterol is delivered to the
liver via IDL and chylomicrons. Unlike other cells, hepatocytes can eliminate
cholesterol by secretion in bile and by conversion to bile acids.
D. Lp(a) Lipoprotein
Lp(a) lipoprotein is
formed from LDL and the (a) protein, linked by a disulfide bridge. The (a)
protein is highly homologous with plasminogen but is not activated by tissue
plasminogen activator. It occurs in a number of isoforms of different molecular
weights. Levels of Lp(a) vary from nil to over 500 mg/dL and are determined
chiefly by genetic factors. Lp(a) can be found in atherosclerotic plaques and
may also contribute to coronary disease by inhibiting thrombolysis. Levels are
elevated in certain inflammatory states. The risk of coronary disease is
strongly related to the level of Lp(a). A common variant (I4399M) in the coding
region is associated with elevated levels.
E. High-Density Lipoproteins
The apoproteins of HDL are secreted by the liver and intestine. Much of the lipid comes from the surface monolayers of chylomi-crons and VLDL during lipolysis. HDL also acquires cholesterol from peripheral tissues, protecting the cholesterol homeostasis of cells.
Free cholesterol is transported from the cell membrane by a transporter, ABCA1,
acquired by a small particle termed prebeta-1 HDL, and then esterified by
lecithin:cholesterol acyltransferase (LCAT), leading to the formation of larger
HDL species. Cholesterol is also exported from macrophages by the ABCG1
transporter to large HDL particles. The cholesteryl esters are transferred to
VLDL, IDL, LDL, and chylomicron remnants with the aid of cholesteryl ester
transfer protein (CETP). Much of the cholesteryl ester thus transferred is
ultimately delivered to the liver by endocytosis of the acceptor lipoproteins.
HDL can also deliver cholesteryl esters directly to the liver via a docking
receptor (scav-enger receptor, SR-BI) that does not cause endocytosis of the
lipoproteins. HDL-C levels relate inversely to risk at the popula-tion level.
Among individuals, the capacity to accept exported cholesterol can vary widely
at identical levels of HDL-C. The abil-ity of peripheral tissues to export
cholesterol via the transporter mechanism and the acceptor capacity of HDL are
emerging as major determinants of coronary atherosclerosis.
Related Topics