Chapter: Basic & Clinical Pharmacology : Introduction to Autonomic Pharmacology
Neurotransmitter Chemistry of the Autonomic Nervous System
NEUROTRANSMITTER CHEMISTRY OF THE
AUTONOMIC NERVOUS SYSTEM
An
important traditional classification of autonomic nerves is based on the
primary transmitter molecules—acetylcholine or norepinephrine—released from
their terminal boutons and vari-cosities. A large number of peripheral ANS
fibers synthesize and release acetylcholine; they are cholinergic fibers; that is, they work by releasing acetylcholine.
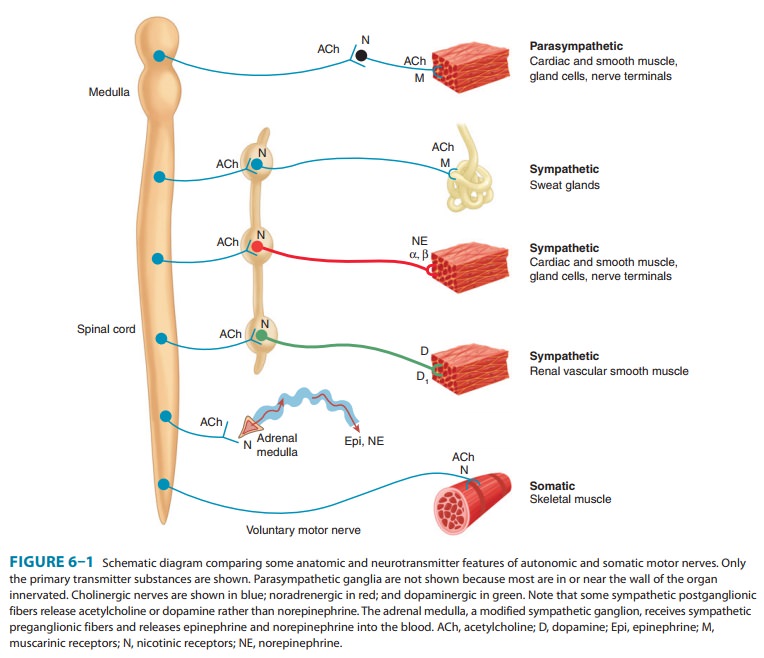
As shown in Figure 6–1, these include all preganglionic efferent autonomic
fibers and the somatic (non-autonomic) motor fibers to skeletal muscle as well.
Thus, almost all efferent fibers leaving the CNS are cholinergic. In addition,
most parasympathetic postganglionic and a few sympatheticpostganglionic fibers
are cholinergic. A significant number of parasympathetic postganglionic neurons
utilize nitric oxide or peptides as the primary transmitter or cotransmitters.
Most
postganglionic sympathetic fibers release norepinephrine (also known as
noradrenaline); they are noradrenergic
(often called simply “adrenergic”) fibers; that is, they work by releasing
norepinephrine (noradrenaline). These transmitter characteristics are presented
schematically in Figure 6–1. As noted, some sympa-thetic fibers release
acetylcholine. Dopamine is a very important transmitter in the CNS, and there
is evidence that it may be released by some peripheral sympathetic fibers.
Adrenal medullary cells, which are embryologically analogous to postganglionic
sym-pathetic neurons, release a mixture of epinephrine and norepi-nephrine.
Finally, most autonomic nerves also release several cotransmitter substances (described in the text that follows),
inaddition to the primary transmitters just described.
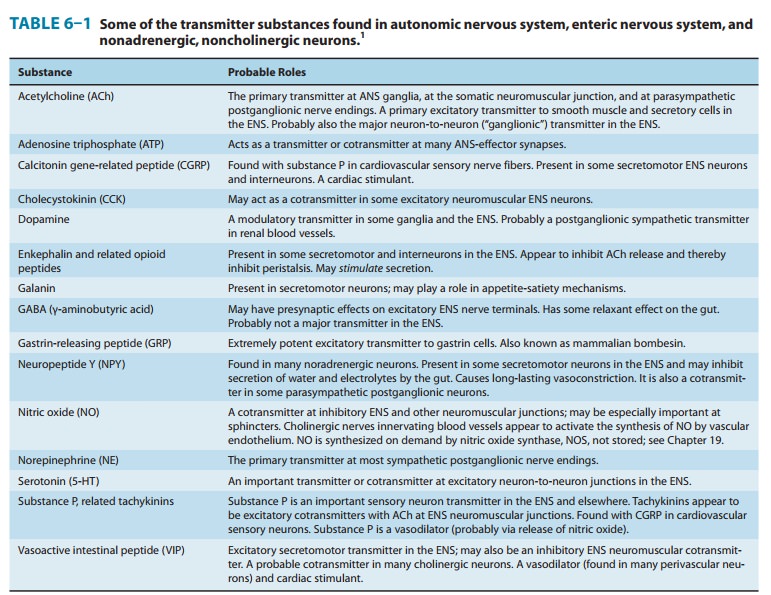
Five
key features of neurotransmitter function provide poten-tial targets for
pharmacologic therapy: synthesis,
storage, release, and termination of
action of the transmitter, and receptoreffects.
These processes are discussed next.
Cholinergic Transmission
The
terminals and varicosities of cholinergic neurons contain large numbers of
small membrane-bound vesicles concentrated near the synaptic portion of the
cell membrane (Figure 6–3) as well as a smaller number of large dense-cored
vesicles located farther from the synaptic membrane. The large vesicles contain
a high concen-tration of peptide cotransmitters (Table 6–1), whereas the
smaller clear vesicles contain most of the acetylcholine. Vesicles are
ini-tially synthesized in the neuron cell body and carried to the termi-nal by
axonal transport. They may also be recycled several times within the terminal.
Vesicles are provided with vesicle-associatedmembrane
proteins (VAMPs), which serve to align them withrelease sites on the inner
neuronal cell membrane and participate in triggering the release of
transmitter. The release site on the inner surface of the nerve terminal membrane
contains synapto-somal nerve-associated
proteins (SNAPs), which interact withVAMPs.
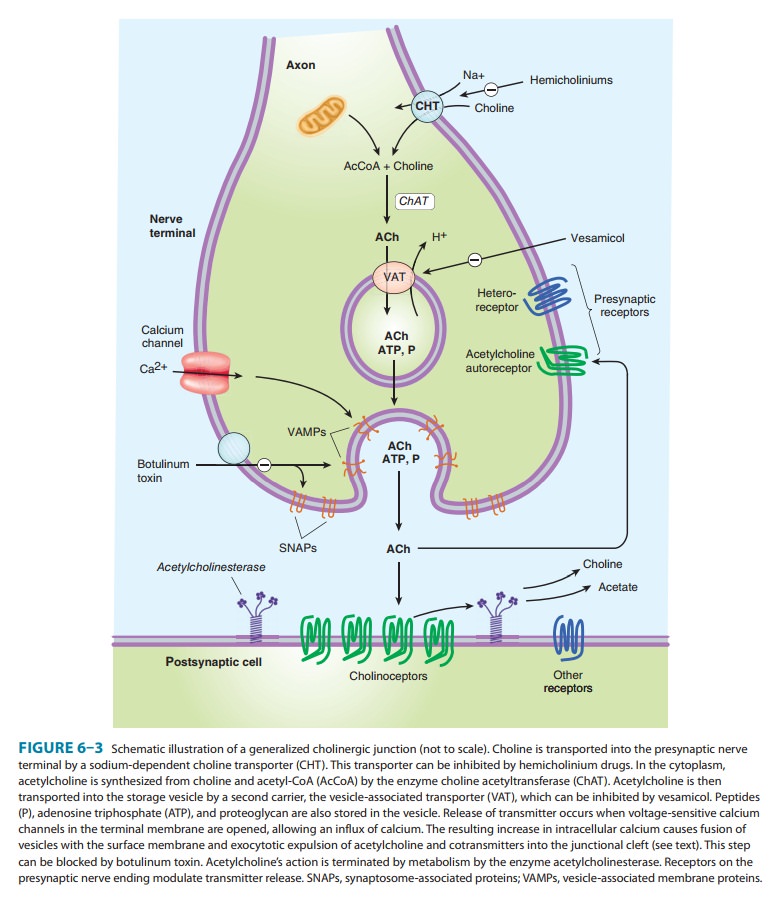
Acetylcholine
is synthesized in the cytoplasm from acetyl-CoA and choline through the
catalytic action of the enzyme cholineacetyltransferase
(ChAT). Acetyl-CoA is synthesized in mito-chondria, which are present in
large numbers in the nerve ending. Choline is transported from the
extracellular fluid into the neuron terminal by a sodium-dependent membrane choline transporter (CHT; Figure 6–3). This symporter can be
blocked by a group of research drugs called hemicholiniums. Once synthesized, acetyl-choline is transported
from the cytoplasm into the vesicles by a vesicle-associated
transporter (VAT) that is driven by protonefflux (Figure 6–3). This
antiporter can be blocked by the research drug vesamicol. Acetylcholine synthesis is a rapid process capable of
supporting a very high rate of transmitter release. Storage of acetylcholine is
accomplished by the packaging of “quanta” of acetylcholine molecules (usually
1000 to 50,000 molecules in each vesicle). Most of the vesicular acetylcholine
(ACh) is bound to negatively chargedvesicular
proteoglycan (VPG).
Vesicles
are concentrated on the inner surface of the nerve terminal facing the synapse
through the interaction of so-called SNARE proteins on the vesicle (a subgroup
of VAMPs called v-SNAREs, especially synaptobrevin)
and on the inside of theterminal cell membrane (SNAPs called t-SNAREs,
especially syntaxin and SNAP-25). Physiologic release of
transmitter fromthe vesicles is dependent on extracellular calcium and occurs
when an action potential reaches the terminal and triggers sufficient
influx
of calcium ions via N-type calcium channels. Calcium interacts with the VAMP synaptotagmin on the vesicle mem-brane
and triggers fusion of the vesicle membrane with the termi-nal membrane and
opening of a pore into the synapse. The opening of the pore and inrush of
cations results in release of the acetylcholine from the proteoglycan and
exocytotic expulsion into the synaptic cleft. One depolarization of a somatic
motor nerve may release several hundred quanta into the synaptic cleft. One
depolarization of an autonomic postganglionic nerve varicosity or terminal
probably releases less and releases it over a larger area. In addition to
acetylcholine, several cotransmitters are released at the same time (Table
6–1). The acetylcholine vesicle release process is blocked by botulinum toxin through the enzymatic
removal of two amino acids from one or more of the fusion proteins.
After
release from the presynaptic terminal, acetylcholine mol-ecules may bind to and
activate an acetylcholine receptor (cholinoceptor).
Eventually (and usually very rapidly), all of the acetylcholine released
diffuses within range of an acetylcholinest-erase
(AChE) molecule. AChE very efficiently splits acetylcholineinto choline and
acetate, neither of which has significant transmit-ter effect, and thereby
terminates the action of the transmitter (Figure 6–3). Most cholinergic
synapses are richly supplied with acetylcholinesterase; the half-life of
acetylcholine molecules in the synapse is therefore very short (a fraction of a
second). Acetylcholinesterase is also found in other tissues, eg, red blood
cells. (Other cholinesterases with a lower specificity for acetylcho-line,
including butyrylcholinesterase [pseudocholinesterase], are found in blood
plasma, liver, glia, and many other tissues.)
Adrenergic Transmission
Adrenergic
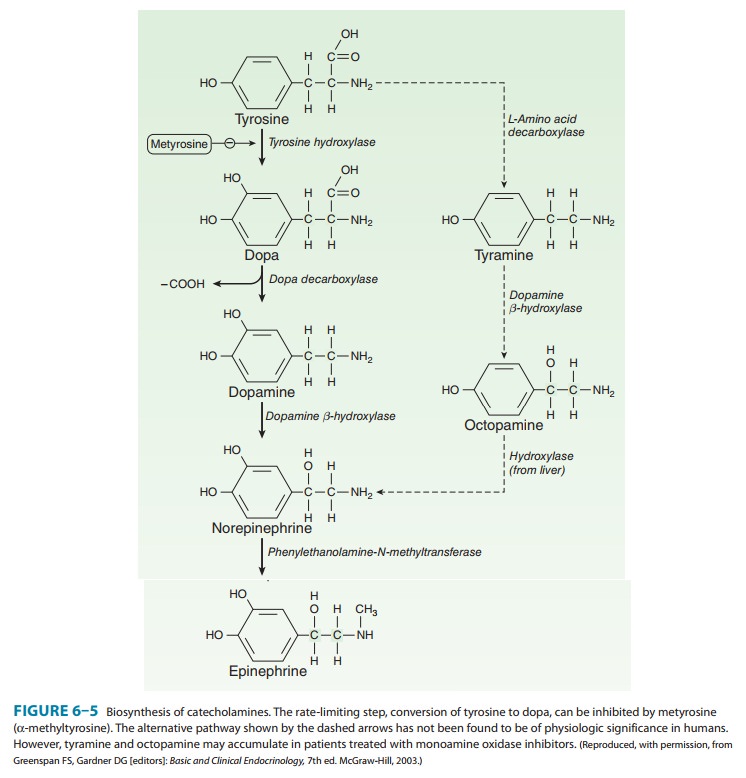
neurons (Figure 6–4) transport a precursor amino acid (tyrosine) into the nerve
ending, then synthesize the catecholamine transmitter (Figure 6–5), and finally
store it in membrane-bound vesicles. In most sympathetic postganglionic
neurons, norepi-nephrine is the final product. In the adrenal medulla and
certain areas of the brain, some norepinephrine is further converted to
epinephrine. In dopaminergic neurons, synthesis terminates with dopamine.
Several processes in these nerve terminals are potential sites of drug action.
One of these, the conversion of tyrosine to dopa, is the rate-limiting step in
catecholamine transmitter syn-thesis. It can be inhibited by the tyrosine
analog metyrosine. A high-affinity
antiporter for catecholamines located in the wall of the storage vesicle (vesicular monoamine transporter, VMAT)
can be inhibited by the reserpine
alkaloids. Reserpine causes depletion of transmitter stores. Another
transporter (norepi-nephrine
transporter, NET) carries norepinephrine and similarmolecules back into the
cell cytoplasm from the synaptic cleft (Figure 6–4; NET). NET is also commonly
called uptake 1 or reuptake 1 and is partially responsible for the termination
of syn-aptic activity. NET can be inhibited by cocaine and tricyclicantidepressant
drugs, resulting in an increase of transmitter activ-ity in the synaptic
cleft (see Box: Neurotransmitter Uptake Carriers).
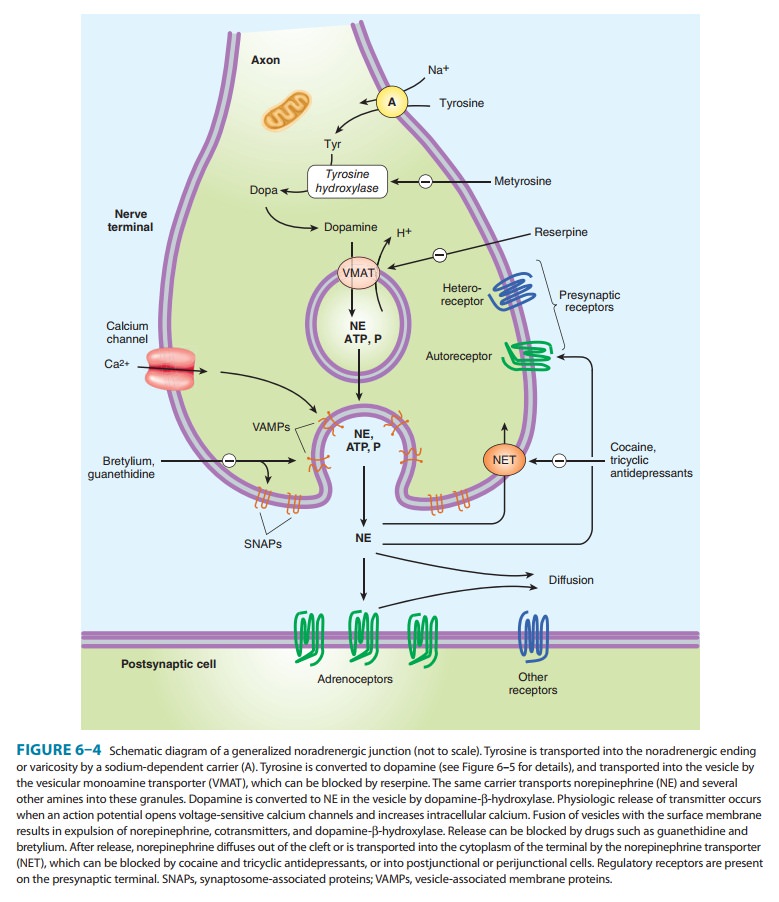
Release
of the vesicular transmitter store from noradrenergic nerve endings is similar
to the calcium-dependent process previ-ously described for cholinergic
terminals. In addition to the pri-mary transmitter (norepinephrine), adenosine
triphosphate (ATP), dopamine-β-hydroxylase, and peptide cotransmitters are
alsoreleased into the synaptic cleft. Indirectly acting and mixed
sym-pathomimetics, eg, tyramine,
amphetamines, and ephedrine, are
capable of releasing stored transmitter from noradrenergic nerve endings by a
calcium-independent process. These drugs are poor agonists (some are inactive)
at adrenoceptors, but they are excellent substrates for monoamine transporters.
As a result, they are avidly taken up into noradrenergic nerve endings by NET.
In the nerve ending, they are then transported by VMAT into the vesicles,
displacing norepinephrine, which is subsequently expelled into the synaptic
space by reverse transport via NET. Amphetamines also inhibit monoamine oxidase
and have other effects that result in increased norepinephrine activity in the
synapse. Their action does not require vesicle exocytosis.
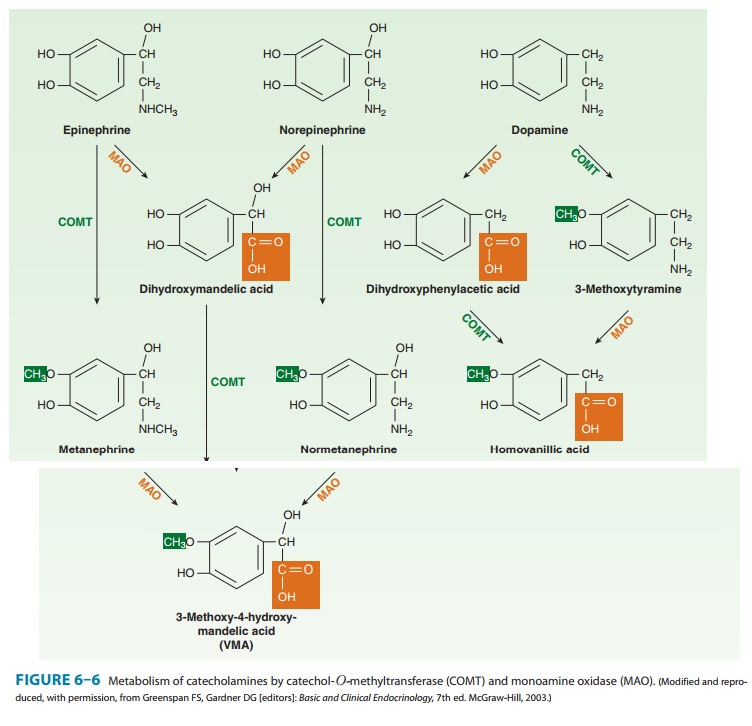
Norepinephrine
and epinephrine can be metabolized by several enzymes, as shown in Figure 6–6.
Because of the high activity of monoamine oxidase in the mitochondria of the
nerve terminal, there is significant turnover of norepinephrine even in the
resting terminal. Since the metabolic products are excreted in the urine, an
estimate of catecholamine turnover can be obtained from labo-ratory analysis of
total metabolites (sometimes referred to as “VMA and metanephrines”) in a
24-hour urine sample. However, metabolism is not the primary mechanism for
termination of action of norepinephrine physiologically released from
noradren-ergic nerves. Termination of noradrenergic transmission results from
two processes: simple diffusion away from the receptor site (with eventual metabolism
in the plasma or liver) and reuptake into the nerve terminal by NET (Figure
6–4) or into perisynaptic glia or other cells.
Cotransmitters in Cholinergic
& Adrenergic Nerves
As
previously noted, the vesicles of both cholinergic and adrener-gic nerves
contain other substances in addition to the primary transmitter, sometimes in
the same vesicles and sometimes in aseparate vesicle population. Some of the
substances identified to date are listed in Table 6–1. Many of these substances
are also primary transmitters in the
nonadrenergic, noncholinergic nervesdescribed in the text that follows. They
appear to play several roles in the function of nerves that release
acetylcholine or norepineph-rine. In some cases, they provide a faster or
slower action to sup-plement or modulate the effects of the primary
transmitter. They also participate in feedback inhibition of the same and
nearby nerve terminals.
Related Topics