Chapter: Plant Biochemistry: Phenylpropanoids comprise a multitude of plant secondary metabolites and cell wall components
Monooxygenases are involved in the synthesis of phenols
Monooxygenases are involved in the synthesis of phenols
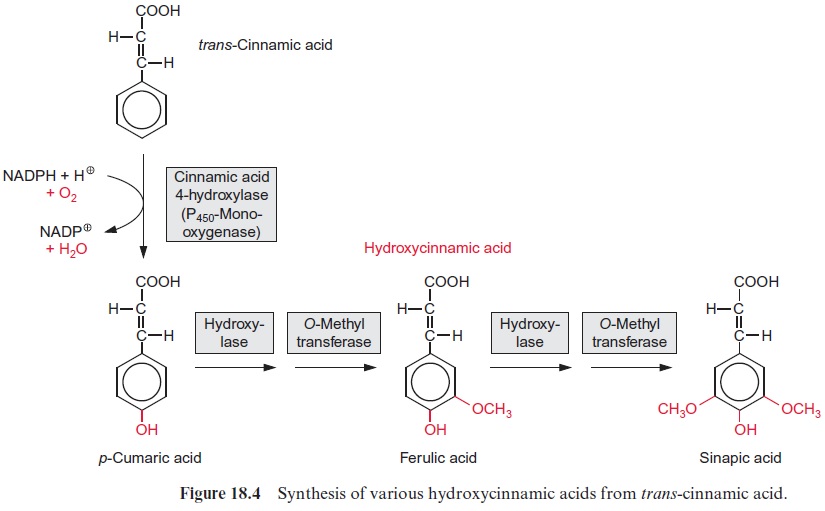
The introduction of the hydroxyl group into the phenyl ring of cinnamic acid (hydroxylation, Fig. 18.4) proceeds via amonooxygenase catalyzed reaction utilizing cytochrome P450 as the O2 binding site according to:
NADPH + H+ + R-CH3 + O2 → NADP + RCH2 –OH + H2O
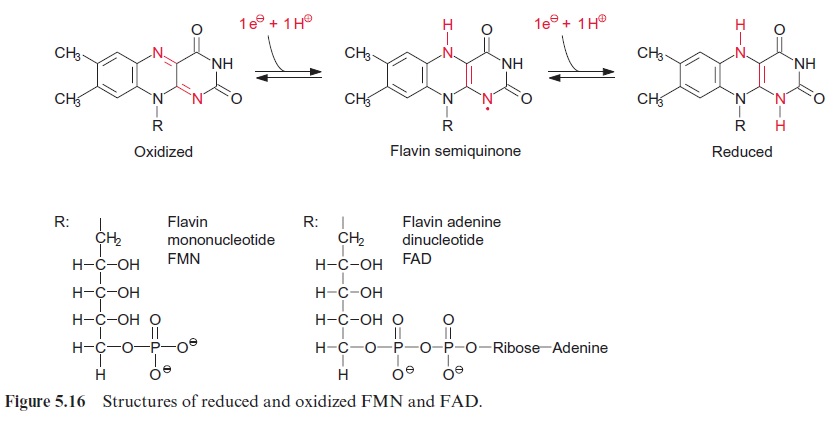
In this reaction, electrons are transferred from NADPH via FAD (Fig. 5.16) to cytochrome-P450 (pigment with absorption maximum at 450 nm) and subsequently to O2. From the O 2 molecule, only one O atom is incor-porated into the hydroxyl group; the remaining O atom is reduced to yield H2O. Since both O atoms are incorporated into two different molecules, it is a monooxygenase reaction. Like cyt-a3 , cyt-P450 can bind CO instead of O2. Therefore, P450-monooxygenases are inhibited by CO.
P450-monooxygenases are widely distributed in the animal and plant kingdoms. Genomic analyses of the model plant Arabidopsis thaliana revealed about 300 different genes that encode P450-proteins. It seems to be the largest gene family in plants. The majority of these proteins is probably involved in the generation of hydroxyl groups for the synthesis of plant hor-mones and secondary metabolites, but they also play an important role in detoxification processes (e.g., the detoxification of herbicides) .
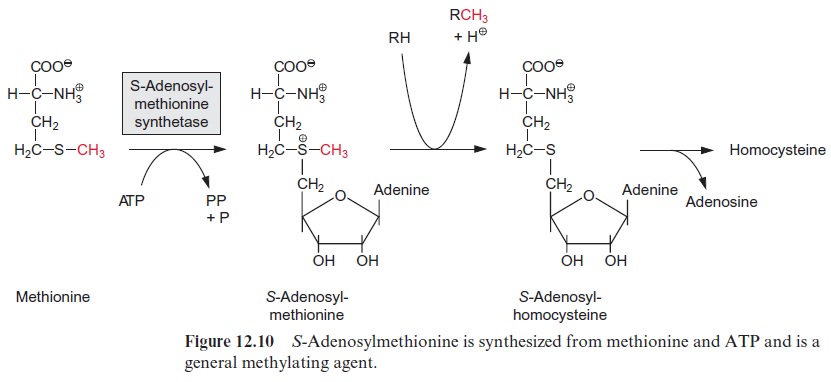
Like all P450-monooxygenases, the cinnamic acid hydroxylase is bound at the membranes of the endoplasmatic reticulum. p-Coumaric acid can be hydroxylated further at positions 3 and 5 by hydroxylases, again by the P450-monooxygenase reaction type. The -OH groups thus generated are methylated mostly via O-methyl transferases with S-adenosylmethionine as the methyl donor (Fig. 12.10). In this way ferulic acid and sinapic acid are synthesized, which, together with p-coumaric acid, are the precursors for the synthesis of lignin .
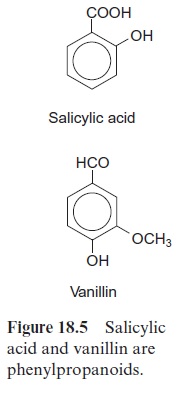
Benzoic acid derivatives, including salicylic acid as well as a derivative of benzaldehyde, vanillin (the aroma substance of vanilla), are formed by cleavage of a C2 fragment from the phenylpropanes (Fig. 18.5). Under the trade name aspirin, the acetyl ester of salicylic acid is widely used as a rem-edy against pain, fever, and other illnesses and is probably the most fre-quently used pharmaceutical worldwide. The name salicylic acid is derived from Salix, the Latin name for willow, since it was first isolated from the bark of the willow tree, where it accumulates in high amounts. Since ancient times, the salicylic acid content of willow bark was used as medi-cine in the Old and New Worlds. In the fourth century BC Hippocrates gave women willow bark to chew to relieve pain during childbirth. Native Americans also used extracts from willow bark as pain killers.
Salicylic acid also affects plants. It has been observed that tobacco plants treated with aspirin or salicylic acid have enhanced resistance to pathogens, such as the Tobacco mosaic virus. Many plants show an increase in their salicylic acid content after being infected by viruses or fungi, but also after being exposed to UV radiation or ozone stress. Salicylic acid is an important signal component of signal transduction chains that lead to the expression of enzymes involved in defense reactions against viruses, bacteria, and fungi. Arabidopsis mutants, which have lost the ability to produce salicylic acid, are more prone to infection, while a dose of salicylic acid can give better protection against pathogens. This principle is now used commercially. A salicylic acid analogue with the trade name Bion (Syngenta) is being sprayed on wheat to prevent mildew infection.
However, salicylic acid not only triggers defense reactions, but can also induce blooming in some plants. By stimulating the mitochondrial alterna-tive oxidase, it activates the production of heat in the spadix of the voodoo lily, emitting a carrion-like stench.
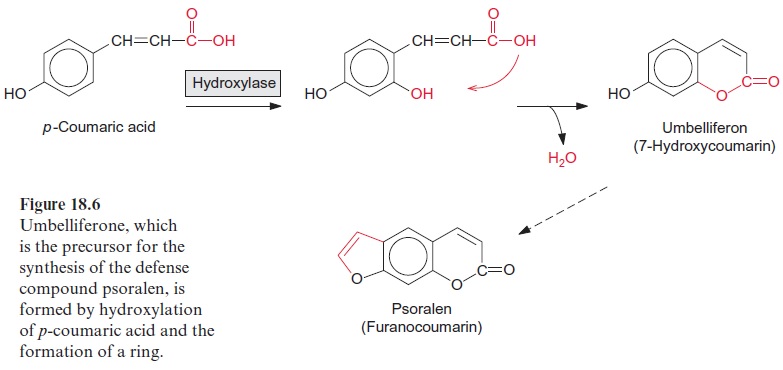
7-Hydroxycoumarin, also called umbelliferone, is synthesized from p-coumaric acid by hydroxylation and the formation of an intermolecular ester, a lactone (Fig. 18.6). The introduction of a C2 group into umbelliferone yields psoralen, a furanocoumarin. Illumination with UV light turns psoralen into a toxic compound. The illuminated psoralen reacts with the pyrimidin bases of the DNA, causing blockage of transcription and DNA repair mech-anisms, which finally results in the death of the cell. Some celery varieties contain very high concentrations of psoralen and caused severe skin inflammation of workers involved in the harvest. Many furanocoumarins have antibiotic properties. In some cases, they are constitu-tive components of the plants, whereas in other cases, they are formed only after infection or wounding as phytoalexins.
Related Topics