Book Back and Important Questions Answers | Chemistry - Metallurgy: Answer the following questions | 12th Chemistry : UNIT 1 : Metallurgy
Chapter: 12th Chemistry : UNIT 1 : Metallurgy
Metallurgy: Answer the following questions
Chemistry : Metallurgy
Answer the following questions:
1. What are the differences between minerals and ores?
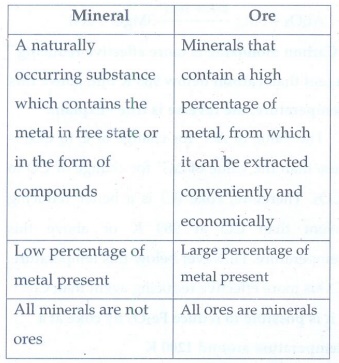
Mineral
•
A naturally occurring substance which contains the metal in free state or in
the form of compounds
•
Low percentage of metal present
•
All minerals are not ores
Ore
•
Minerals that contain a high percentage of metal, from which it can be
extracted conveniently and economically
•
Large percentage of metal present
•
All ores are minerals
2. What are the various steps involved in extraction of pure metals from their ores?
(i)
Concentration of the ore
(ii)
Extraction of crude metal
(iii)
Refining of crude metal
3. What is the role of Limestone in the extraction of Iron from its oxide Fe2O3 ?
The
silica gangue present in the iron ore is acidic in nature. Silica removed by
using basic flux, limestone (CaO). The limestone combines with silica gangue to
form calcium silicate (slag). The slag was removed.
CaCO3
___Δ__→ CaO + CO2
CaO(s) [Flux]
+ SiO2(S)
[Gangue] → CaSiO3(S)
[Slag]
Flux + Gangue → Slag
4. Which type of ores can be concentrated by froth floatation method? Give two examples for such ores.
Sulphide
ores can be concentrated by froth flotation method.
Example: Zinc
blende (ZnS), Silver glance (Ag2S)
5. Out of coke and CO, which is better reducing agent for the reduction of ZnO? Why?
Coke
(C) is a better reducing agent for the reduction of ZnO. From the Ellingham
diagram, the free energy for the formation of CO from C is lower at above 1120
K while that of CO2 from carbon is lower at above 1320 K than free
energy of formation of ZnO. However, the free energy of formation of CO2 from
CO is always higher than that of ZnO.
6. Describe a method for refining nickel.
The
impure nickel is heated in a stream of carbon monoxide at around 350 K. The
nickel reacts with the CO to form a highly volatile nickel tetracarbonyl. The
solid impurities are left behind.
Ni
(s) + 4 CO (g) → Ni(CO)4 (g)
The
nickel tetracarbonyl heated around 460 K, the complex decomposes to give pure
nickel.
Ni(CO)4 (g) → Ni (s) + 4 CO (g)
7. Explain zone refining process with an example using the Ellingham diagram given below.
Zone refining process:
i)
This method is based on the principle of fractional crystallisation. When an
impure metal is melted and allowed to solidify, the impurities are more soluble
in the melt than in the solid state metal.
ii)
The impure metal is taken in the form of a rod. One end of the rod is heated
using a mobile induction heater which results in melting of the metal on that
portion of the rod.
iii)
When the heater is slowly moved to the other end, the pure metal crystallises
while the impurities will move on to the adjacent molten zone formed. As the
heater moves further away, the impurities also moves along with it.
iv)
The process is repeated several times to achieve the pure element.
v)
This process is carried out in an inert gas atmosphere to prevent the oxidation
of metals.
Example:
Elements
such as germanium (Ge), silicon (Si) and galium (Ga) that are used as
semiconductor are refined using this process.
8. (A) Predict the conditions under which
(i) Aluminium might be expected to reduce magnesia.
In
the Ellingham diagram, above 1623 K, the ∆G° value for Al2O3
is more negative than that of MgO. Thus ∆G° of the reaction is negative.
Therefore
above 1623 K, Al can reduce MgO to Mg.
3MgO + 2Al ___1623 K__→ Al2O3
+ 3Mg
(ii) Magnesium could reduce alumina.
In
the Ellingham diagram, below 1623 K the ∆G° value of Al2O3,
is less negative than that of MgO. Thus, ∆G°of the reaction is negative.
Therefore, below 1623 K, Mg can reduce Al2O3 to Al.
Al2O3 + 3Mg ___below 1623__→ 3MgO +
2Al
(B) Carbon monoxide is more effective reducing agent than carbon below 983K but, above this temperature, the reverse is true –Explain.
The
value of ∆G° for change of C to CO2 is less than the value of ∆G°
for change of CO to CO2. Therefore, coke (C) is a better reducing
agent than CO at 983 K or above this temperature. However below this
temperature, CO is more effective reducing agent than C.
(C) it is possible to reduce Fe2 O3 by coke at a temperature around 1200K
Yes,
it is possible to reduce Fe2O3 by coke at a temperature
around 1200K . Ellingham diagram for the formation of FeO and CO intersects
around 1000 K. Below this temperature, the carbon line lies above the iron line
which indicates that FeO is more stable and the reduction is thermodynamically
not feasible. Around 1200 K carbon line lies below the iron line and hence,
coke can be used as reducing agent.
Fe2O3
+ C → 2Fe + 3CO
9. Give the uses of zinc.
• Metallic zinc is used in
galvanising metals.
• It is used to produce
die-castings in the automobile, electrical and hardware industries.
• Zinc oxide is used in the
manufacture of many products such as paints, rubber, cosmetics,
pharmaceuticals, plastics, inks, batteries, textiles and electrical equipment.
• Zinc sulphide is used in making
luminous paints, fluorescent lights and X-ray screens.
• Brass an alloy of zinc is used in
water valves and communication equipment as it is highly resistant to
corrosion.
10. Explain the electrometallurgy of aluminium.
Hall-Heroult
process:
Cathode: Iron
tank lined with carbon
Anode: The
carbon blocks immersed in the electrolyte
A
20% solution of alumina, obtained from the bauxite ore is mixed with molten
cyrolite and is taken in the electrolysis chamber.
About
10% calcium chloride is also added to the solution, which lowers the melting
point of the mixture.
The
fused mixture is maintained at a temperature of above 1270 K.
Ionisation
of alumina
Al2O3
→ 2Al3+ + 3O2−
Reaction
at cathode
Al3+
(melt) + 3e− → Al (1)
Reaction
at anode
2O2−
(melt) → O2 + 4e−
Carbon
anode consumed slowly.
C(s)
+ O2− (melt) → CO + 2e−
C(s)
+ 2O 2− (melt) → CO2 + 4e−
The
pure aluminium is formed at the cathode and settles at the bottom. The net
electrolysis reaction is
4Al3+
(melt) + 6O2(melt) + 3C(s) → 4Al(ℓ)
+ 3CO2(g)
11. Explain the following terms with suitable examples.
(i) Gangue (ii) slag
Gangue:
The
ores are associated with nonmetallic impurities, rocky materials and siliceous
matter which are collectively known as gangue.
Example:
The
silica gangue present in the iron ore
Slag:
When
gangue present in the roasted or calcined ore combined with the flux forms a
fusible material called slag.
Example:
Silica
gangue in the iron ore removed by using limestone (CaO). The slag was removed.
CaO(s) [Flux]
+ SiO2(s) [Gangue]
→ CaSiO3(s) [Slag]
Flux + Gangue → Slag
12. Give the basic requirement for vapour phase refining.
• The metal is treated with a
suitable reagent it should form a volatile compound with the metal.
• The volatile compound is easily
decomposed to give the pure metal.
13. Describe the role of the following in the process mentioned.
(i) Silica in the extraction of copper.
(ii) Cryolite in the extraction of aluminium.
(iii) Iodine in the refining of Zirconium.
(iv) Sodium cyanide in froth floatation.
(i) Silica in the
extraction of copper.
Silica
is used as flux material in the extraction of copper.
The
concentrated ore is heated with an acidic flux silica. The ferrous oxide formed
due to melting is basic in nature and it combines with silica to form ferrous
silicate (slag).
FeO
+ SiO2 → FeSiO3
(ii) Cryolite in
the extraction of aluminium.
(a)
The melting point of alumina is very high.
Hence
it is mixed with cryolite (Na3AlF6) which lowers its
melting point
(b)
Cryolite increase the electrical conductivity of alumina.
(c)
The function of cryolite is to lower the fusion temperature.
(iii) Iodine in the
refining of Zirconium.
The
impure zirconium metal is heated with iodine at a temperature of 550 K to form
the volatile zirconium tetra-iodide. The impurities are not reacting with
iodine. The volatile tetraiodide vapour is passed over a tungsten filament at
high temperature and pure zirconium was obtained.
Zr(s)
+ 2I2 (s) __ 550 K_→
ZrI4 (vapour)
ZrI4
(vapour) ___1800 K_→ Zr (s) + 2I2 (s)
(iv) Sodium cyanide
in froth floatation
Sodium
cyanide (NaCN) is added to depress the floatation property.
When
a sulphide ore of a metal contains other metal sulphides as impurities,
depressing agent sodium cyanide is used to selectively prevent other metal
sulphides from coming to the froth.
14. Explain the principle of electrolytic refining with an example.
The
crude metal is refined by electrolysis.
Anode - Impure
metal rod
Cathode - Thin
strips of pure metal
The
metal dissolves from the anode, pass into the solution while the same amount of
metal ions from the solution will be deposited at the cathode. The insoluble
impurities in the anode settles at the bottom of the anode as anode mud.
Example:
Electrolytic refining of silver
Cathode
: Pure silver
Anode
: Impure silver rods
Electrolyte
: Silver nitrate in HNO3
Reaction
at cathode
Ag+
(aq) + e− → Ag(s)
Reaction
at anode
Ag(s)
→ Ag+ (aq) + e−
During
electrolysis, at the anode the silver atoms lose electrons and enter the
solution. The positively charged silver cations migrate towards the cathode and
get discharged by gaining electrons and deposited on the cathode. The
impurities settle down at the bottom of anode as anode mud.
15. The selection of reducing agent depends on the thermodynamic factor: Explain with an example.
The
extraction of metals from their oxides can be carried out by using different
reducing agents.
2/y
MxOy(s) → 2x / y M(s) + O2(g)
The
above reduction may be carried out with carbon. The reducing agent carbon may
be oxidised to either CO or CO2.
C
+ O2 → CO2
2
C + O2 → 2 CO
If
carbon monoxide is used as a reducing agent, it is oxidised to CO2
as follows
2CO
+ O2 → 2 CO2
A
suitable reducing agent is selected based on the thermodynamic considerations.
The change in free energy (∆G) should be negative, for a spontaneous reaction.
Therefore, thermodynamically, the reduction of metal oxide with a given
reducing agent can occur if the free energy change for the coupled reaction is
negative. Hence, the reducing agent is selected in such a way that it provides
a large negative ∆G value for the coupled reaction.
16. Give the limitations of Ellingham diagram.
i)
Ellingham diagram is constructed based only on thermodynamic considerations. It
gives information about the thermodynamic feasibility of a reaction. It does
not tell anything about the rate of the reaction. Moreover, it does not give
any idea about the possibility of other reactions that might be taking place.
ii)
The interpretation of ∆G is based on the assumption that the reactants are in
equilibrium with the product which is not always true.
17. Write a short note on electrochemical principles of metallurgy.
The
reduction of oxides of active metals by carbon is thermodynamically not feasible.
Such metals are extracted by using electrochemical methods. The metal salts are
taken in a fused form or in solution form. The metal ion present can be reduced
by treating it with some suitable reducing agent or by electrolysis.
Gibbs
free energy change for the electrolysis process is given by the following
expression
∆G°
= −nFE°
Where
n is number of electrons involved in the reduction process
F
is the Faraday
E°
is the electrode potential of the redox couple.
If
E° is positive then the ∆G is negative and the reduction is spontaneous. When a
more reactive metal is added to the solution containing the relatively less
reactive metal ions, the more reactive metal will go into the solution.
For
example,
Cu
(s) + 2Ag+ (s) → Cu2+ (aq) + 2Ag(s)
Cu+2 (aq) + Zn(s) → Cu (s) + Zn+2 (aq)
EVALUATE YOURSELF:
1. Write the
equation for the extraction of silver by leaching with sodium cyanide and show
that the leaching process is a redox reaction.
In
the metallurgy of silver metal is leached with a dilute solution of NaCN in the
presence of air (O2).
4Ag
+ 8CN− + 2H2O + O2 → 4[Ag(CN)2]+
4OH−
In
this reaction, Ag → Ag+ oxidation number of Ag increases from 0 to
+1, hence oxidation
O2
→ OH− (oxidation number of oxygen decreases from 0 to −2, hence
reduction)
Hence
Leaching of silver is a redox reaction.
2. Magnesite
(Magnesium carbonate) is calcined to obtain magnesia, which is used to make
refractory bricks. Write the decomposition reaction.
Magnesite
(Magnesium carbonate) is heated in the absence of oxygen decomposes to form
Magnesium oxide (Magnesia)
MgCO3
→ MgO + CO2 ↑
3. Using Ellingham
diagram indicate the lowest temperature at which ZnO can be reduced to Zinc
metal by carbon. Write the overall reduction reaction at this temperature.
Ellingham
diagram for the formation of ZnO and CO intersects around 1233 K below this
temperature, Carbon line lies above Zinc line. Hence ZnO is more stable than CO
so the reduction is thermodynamically not feasible at this temperature range.
However
above 1233 K carbon line lies below the zinc line, hence carbon can be used as
a reducing agent above 1233 K.
2Zn
+ O2 → 2ZnO ………….. (1)
2C
+ O2 → 2CO …………… (2)
Reversing
(1) and adding with equation (2)
2ZnO
→ 2Zn + O2
2C
+O2 → 2CO
2ZnO
+ 2C → 2Zn + 2CO
ZnO
+ C → Zn + CO
4. Metallic sodium
is extracted by the electrolysis of brine (aq.NaCl). After electrolysis the
electrolytic solution becomes basic in nature. Write the possible electrode
reactions.
Sodium
metal is prepared by Down's process. This involves the electrolysis of fused
NaCl and CaCl2 at 873K. During electrolysis sodium is discharged at
the cathode and Cl2 is obtained at the anode.
NaCl(l)
→ Na+(melt) + Cl− (melt)
Cathode
: Na+(melt) + e− → Na(s)
Anode
: 2Cl− (aq) → Cl2(g) + 2e−
If
an aqueous solution of NaCl is electrolysed, H2 is evolved at
cathode and Cl2 is evolved at anode. NaOH is obtained in the
solution.
NaCl(aq)
___Electrolysis_→ Na+(aq) +
Cl− (aq)
Cathode
: 2H2O(I) + 2e− → H2(g) + 2OH−
(aq)
Anode
: Cl− (aq) → 1/2 Cl2(g) + 2e−
Na+
and OH− ions to form NaOH
Hence solution is basic in nature.
Related Topics