Metallurgy | Chemistry - Extraction of crude metal | 12th Chemistry : UNIT 1 : Metallurgy
Chapter: 12th Chemistry : UNIT 1 : Metallurgy
Extraction of crude metal
Extraction
of crude metal
The extraction of crude
metals from the concentrated ores is carried out in two steps namely, (i)
conversion of the ore into oxides of the metal of interest and (ii) reduction
of the metal oxides to elemental metals. In the concentrated ore, the metal
exists in positive oxidation state and hence it is to be reduced to its
elemental state. We can infer from the principles of thermodynamics, that the
reduction of oxide is easier when compared to reduction of other compounds of
metal and hence, before reduction, the ore is first converted into the oxide of
metal of interest.
Let us discuss some of
the common methods used to convert the concentrated ore into the oxides of the
metal of interest.
1. Conversion of ores into oxides
Roasting
Roasting is the method,
usually applied for the conversion of sulphide ores into their oxides. In this
method, the concentrated ore is oxidised by heating it with excess of oxygen in
a suitable furnace below the melting point of the metal.
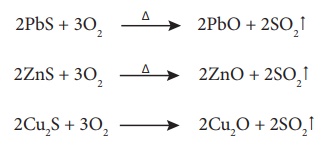
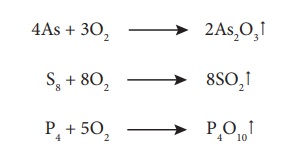
Roasting also removes
impurities such as arsenic, sulphur, phosphorous by converting them into their
volatile oxides.
For example
Calcination
Calcination is the
process in which the concentrated ore is strongly heated in the absence of air.
During this process, the water of crystallisation present in the hydrated oxide
escapes as moisture. Any organic matter (if present) also get expelled leaving
behind a porous ore. This method can also be carried out with a limited supply
of air.
For examples,
During calcination of
carbonate ore, carbon dioxide is expelled
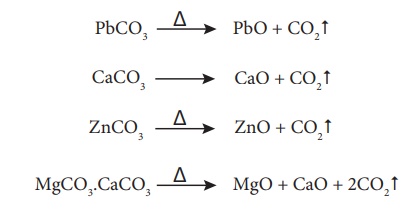
During calcination of
hydrated ore, the water of hydration is expelled as vapour

2. Reduction of metal oxides
Metal oxide can be
reduced to crude metal by using a suitable reducing agent like carbon, carbon
monoxide, hydrogen, aluminium and other reactive metals such as sodium
etc...The choice of reducing agent depends on the nature of the metal. For
example, carbon cannot be used as a reducing agent for the reactive metals such
as sodium, potassium, aluminium etc...Similarly CO cannot be used to reduce
oxides such as ZnO, Al2O3. Later in this,we study
selection of suitable reducing agents by applying Ellingham diagram.
Smelting
In this method, a flux
(a chemical substance that forms an easily fusible slag with gangue) and a
reducing agent such as carbon, carbon monoxide (or) aluminium is added to the
concentrated ore and the mixture is melted by heating at an elevated
temperature (above the melting point of the metal) in a smelting furnace. For
example the oxide of iron can be reduced by carbon monoxide as follows.
Fe2O3
(s) + 3CO (g) → 2Fe (s) + 3CO2 (g) ↑
In this extraction, a
basic flux, limestone (CaO) is used. Since the silica gangue present in the ore
is acidic in nature, the limestone combines with it to form calcium silicate
(slag).
CaO(s) + SiO2 (s) → CaSiO3
(s)
Flux + Gangue → Slag
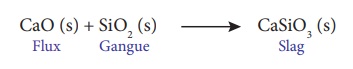
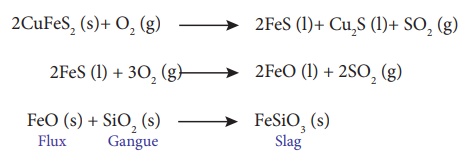
In the extraction of
copper from copper pyrites, the concentrated ore is heated in a reverberatory
furnace after mixing with silica, an acidic flux. The ferrous oxide formed due
to melting is basic in nature and it combines with silica to form ferrous
silicate (slag). The remaining metal sulphides Cu2S and FeS are mutually
soluble and form a copper matte.
2CuFeS2 (s)+ O2 (g) → 2FeS (l)+ Cu2S (l)+ SO2 (g)
2FeS (l) + 3O2 (g) → 2FeO (l) + 2SO2 (g)
FeO (s) + SiO2 (s) → FeSiO3 (s) { Flux + Gangue → Slag }
The matte is separated
from the slag and fed to the converting furnace. During conversion, the FeS
present in the matte is first oxidised to FeO. This is removed by slag
formation with silica. The remaining copper sulphide is further oxidised to its
oxide which is subsequently converted to metallic copper as shown below.
2Cu2S (l,s)
+ 3O2 (g) → 2Cu2O (l,s) + 2SO2 (g)
2Cu2O (l)
+ Cu2S (l) → 6Cu (l) + SO2 (g)
The metallic copper is
solidified and it has blistered appearance due to evolution of SO2
gas formed in this process. This copper is called blistered copper.
Reduction by carbon:
In this method the oxide
ore of the metal is mixed with coal (coke) and heated strongly in a furnace
(usually in a blast furnace). This process can be applied to the metals which
do not form carbides with carbon at the reduction temperature.
Examples:
ZnO (s)+ C (s) → Zn (s) + CO (g) ↑
Mn3O4 (s) + 4C (s) → 3Mn (s) + 4CO (g) ↑
Cr2O3 (s) + 3C (s) → 2Cr (s) + 3CO (g) ↑
Reduction by hydrogen:
This method can be
applied to the oxides of the metals (Fe, Pb, Cu) having less electro-positive
character than hydrogen.
Ag2O (s)+ H2 (g) → 2Ag (s) + H2O (l)
Fe3O4 (s) + 4H2 (g) → 3Fe (s) + 4H2O (l)
Nickel oxide can be
reduced to nickel by using a mixture of hydrogen and carbon monoxide (water
gas)
2NiO (s) + CO (g) + H2
(g) → 2Ni (s) + CO2 (g) + H2O (l)
Reduction by metal:
Metallic oxides such as
Cr2O3 can be reduced by an aluminothermite process. In
this process, the metal oxide is mixed with aluminium powder and placed in a
fire clay crucible. To initiate the reduction process, an ignition mixture
(usually magneisium and barium peroxide) is used.
BaO2 + Mg → BaO
+ MgO
During the above
reaction a large amount of heat is evolved (temperature up to 2400°C, is
generated and the reaction enthalpy is : 852 kJ mol-1) which facilitates the
reduction of Cr2O3 by aluminium power.
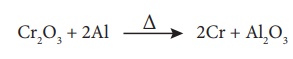
Active metals such as
sodium, potassium and calcium can also be used to reduce the metal oxide
B2O3
+ 6Na → 2B + 3Na2O
Rb2O3
+ 3Mg → 2Rb + 3MgO
TiO2 + 2Mg → Ti + 2MgO
Auto-reduction:
Simple roasting of some
of the ores give the crude metal. In such cases, the use of reducing agents is
not necessary. For example, mercury is obtained by roasting of its ore cinnabar
(HgS)
HgS (s) + O2 →
(g) Hg (l) + SO2↑
Related Topics