Metallurgy | Chemistry - Concentration of ores | 12th Chemistry : UNIT 1 : Metallurgy
Chapter: 12th Chemistry : UNIT 1 : Metallurgy
Concentration of ores
Concentration
of ores
Generally, the ores are
associated with non-metallic impurities, rocky materials and siliceous matter
which are collectively known as gangue. The preliminary step in metallurgical
process is removal of these impurities. This removal process is known as
concentration of ore. It increases the concentration of the metal of interest
or its compound in the ore. Several methods are available for this process and
the choice of method will depend on the nature of the ore, type of impurity and
environmental factors. Some of the common methods of ore concentration are
discussed below.
1. Gravity separation or Hydraulic wash
In this method, the ore
having high specific gravity is separated from the gangue that has low specific
gravity by simply washing with running water. Ore is crushed to a finely
powdered form and treated with rapidly flowing current of water. During this
process the lighter gangue particles are washed away by the running water. This
method is generally applied to concentrate the native ore such as gold and
oxide ores such as haematite (Fe2O3), tin stone (SnO2)
etc.
2. Froth flotation
This method is commonly
used to concentrate sulphide ores such as galena (PbS), zinc blende (ZnS)
etc... In this method, the metallic ore particles which are preferentially
wetted by oil can be separated from gangue.
In this method, the
crushed ore is suspended in water and mixed with frothing agent such as pine
oil, eucalyptus oil etc.
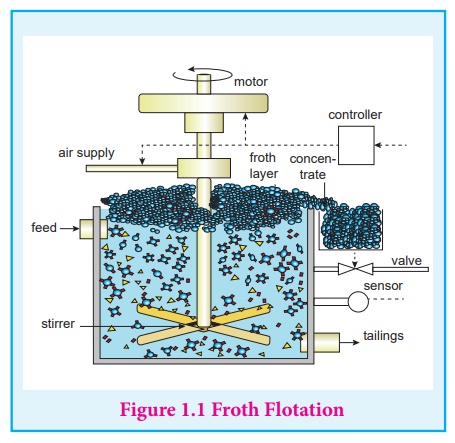
A small quantity of sodium
ethyl xanthate which acts as a collector is also added. A froth is generated by
blowing air through this mixture. The collector molecules attach to the ore
particle and make them water repellent. As a result, ore particles, wetted by
the oil, rise to the surface along with the froth. The froth is skimmed off and
dried to recover the concentrated ore. The gangue particles that are
preferentially wetted by water settle at the bottom.
When a sulphide ore of a
metal of interest contains other metal sulphides as impurities, depressing
agents such as sodium cyanide, sodium carbonate etc are used to selectively
prevent other metal sulphides from coming to the froth. For example, when
impurities such as ZnS is present in galena (PbS), sodium cyanide (NaCN) is
added to depresses the flotation property of ZnS by forming a layer of zinc
complex Na2[Zn(CN)4] on the surface of zinc sulphide.
3. Leaching
This method is based on
the solubility of the ore in a suitable solvent and the reactions in aqueous
solution. In this method, the crushed ore is allowed to dissolve in a suitable
solvent, the metal present in the ore is converted to its soluble salt or
complex while the gangue remains insoluble. The following examples illustrate
the leaching processes.
Cyanide leaching
Let us consider the
concentration of gold ore as an example. The crushed ore of gold is leached
with aerated dilute solution of sodium cyanide. Gold is converted into a
soluble cyanide complex. The gangue, aluminosilicate remains insoluble.
4Au (s) + 8CN-
(aq) + O2 (g) + 2H2O (l) → 4[Au(CN)2]-
(aq) + 4OH-(aq)
Recovery of metal of interest from the complex by reduction:
Gold can be recovered by
reacting the deoxygenated leached solution with zinc. In this process the gold
is reduced to its elemental state (zero oxidation sate) and the process is
called cementation.
Zn (s) + 2[Au(CN)2]-
(aq) → [Zn(CN)4]2-(aq) + 2Au (s)
Ammonia leaching
When a crushed ore
containing nickel, copper and cobalt is treated with aqueous ammonia under
suitable pressure, ammonia selectively leaches these metals by forming their
soluble complexes viz. [Ni(NH3)6]2+, [Cu(NH3)4]2+
, and [Co(NH3)5H2 O]3+ respectively
from the ore leaving behind the gangue, iron(III) oxides/hydroxides and
aluminosilicate.
Alkali leaching
In this method, the ore
is treated with aqueous alkali to form a soluble complex. For example, bauxite,
an important ore of aluminum is heated with a solution of sodium hydroxde or
sodium carbonate in the temperature range 470 - 520 K at 35 atm to form soluble
sodium meta-aluminate leaving behind the impurities, iron oxide and titanium
oxide.
Al2O3
(s) + 2NaOH (aq) + 3H2O (l) → 2Na[Al(OH)4] (aq)
The hot solution is
decanted, cooled, and diluted. This solution is neutralised by passing CO2
gas, to the form hydrated Al2O3 precipitate.
2Na[Al(OH)4]
(aq) + CO2 (g) → Al2O3.xH2O (s) +
2NaHCO3 (aq)
The precipitate is
filtered off and heated around 1670 K to get pure alumina Al2O3.
Acid leaching
Leaching of sulphide
ores such as ZnS, PbS etc., can be done by treating them with hot aqueous
sulphuric acid.
2ZnS (s) + 2H2SO4
(aq) + O2(g) → 2ZnSO4 (aq) + 2S (s) + H2O
In this process the
insoluble sulphide is converted into soluble sulphate and elemental sulphur.
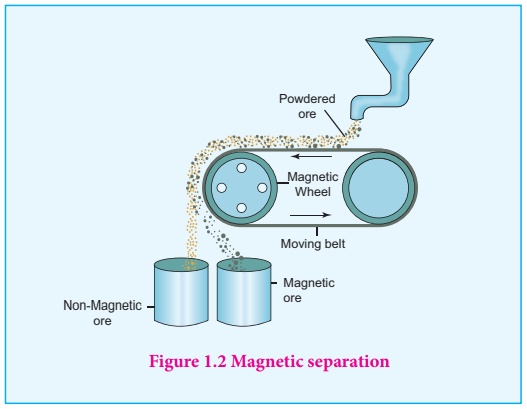
4. Magnetic separation
This method is
applicable to ferromagnetic ores and it is based on the difference in the
magnetic roperties of the ore and the impurities. For example tin stone can be
separated from the wolframite impurities which is magnetic. Similarly, ores
such as chromite, pyrolusite having magnetic property can be removed from the
non magnetic siliceous impurities. The crushed ore is poured on to an
electromagnetic separator consisting of a belt moving over two rollers of which
one is magnetic. The magnetic part of the ore is attracted towards the magnet
and falls as a heap close to the magnetic region while the nonmagnetic part
falls away from it as shown in the figure 1.2.
Related Topics