Chapter: Aquaculture Engineering : Instrumentation and Monitoring
Measuring total gas pressure and nitrogen saturation - Instruments for measuring water quality in Aquaculture
Measuring total gas pressure and nitrogen saturation
The total gas pressure in the water is measured mainly to find not only the total pressure, but also the amount and saturation of dissolved nitrogen gas (N2). If the saturation of nitrogen in the water is above 100%, the fish may suffer from gas bubble disease. This is more critical in fry stage fish than in adult fish. In salmonids problems have been observed when saturation is over 102%, but it is recommended that saturation be maintained below
100.5%. Marine fish fry has been shown to be very sensitive for supersaturation of nitrogen. Problems may also occur if the total gas pressure is too high and there are some indications that above 100% total pressure may be detrimental.
One method to measure the total gas pressure in the water is to use a saturometer (saturation meter) (Fig. 19.3). The main part of the instrument is a small silicon tube into which the dissolved gases from the water pass (the silicon acts as a mem-brane) and become enclosed. A pressure meter is attached to the tube and the measurement is carried out by determining the difference in pres-sure between the local atmospheric pressure and the pressure inside the silicon tube.

Before using the saturometer, the pressure inside the cylinder must be equalized to the surrounding environmental pressure. This is carried out by placing the probe in the air for some minutes before placing it in the water. A perforated cover sur-rounds the cylinders and a manual pump is used to ensure water flow past the cylinder. The instrument has to stay in the water for 5–10 minutes before a reading can be taken.
In order to determine the total gas pressure when using a saturometer the following equation must be employed:
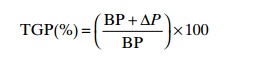
Where:
TGP = total gas pressure of dissolved gases
BP = local barometric pressure (normally read in mmHg (mercury))
∆P= pressure difference (read from the saturometer) between total gas pressure in the water and local barometric pressure (mmHg).
When calculating the nitrogen pressure, which is a critical factor for avoiding gas bubble disease, the following equation may be used:
N2 (%) = [BP +∆P− ((O2/βO2) × 0.5318 − PH2O)/((BP − PH2O) × 0.7902)] × 100
Where:
N2 = partial pressure of nitrogen gas in the water
(percentage nitrogen saturation)
BP = local barometric pressure (mmHg)
∆P= difference between total gas pressure and local barometric pressure, measured by a saturometer (mmHg)
O2 = oxygen concentration in the water measured
by an oxygen meter (mg/L)
βO2 = Bunsen’s coefficient for oxygen (see Appendix 8.2)
PH2O=partial pressure of the water vapour (mmHg)
The two numbers in the equation are conversion factors.
There are also other instruments available for monitoring the nitrogen gas saturation which are simpler to use.
Related Topics