Chapter: Basic & Clinical Pharmacology : Introduction to the Pharmacology of Central Nervous System (CNS) Drugs
Ion Channels & Neurotransmitter Receptors
ION CHANNELS &
NEUROTRANSMITTER RECEPTORS
The
membranes of nerve cells contain two types of channels defined on the basis of
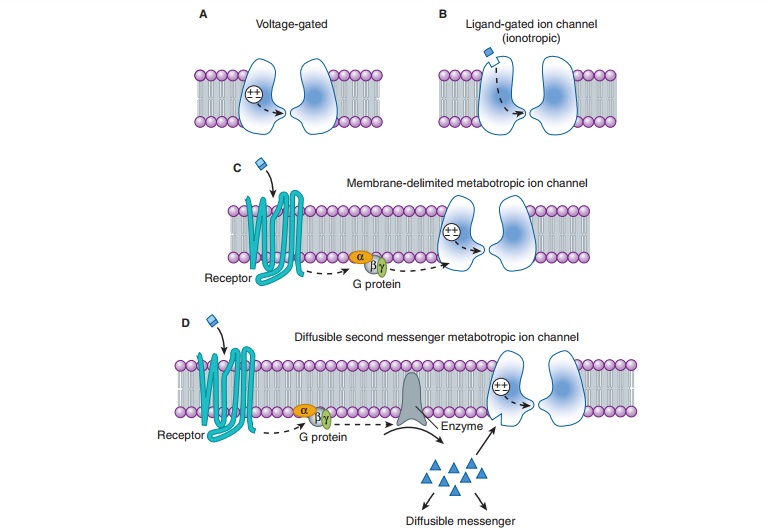
the mechanisms controlling their gating (opening and closing): voltage-gated and ligand-gated channels (Figure 21–2A and B). Voltage-gated channels
respond to changes in the membrane potential of the cell. The voltage-gated
sodium channel described for the heart is an example of the first type of
channel. In nerve cells, these channels are concen-trated on the initial segment
and the axon and are responsible for the fast action potential, which transmits
the signal from cell body to nerve terminal. There are many types of
voltage-sensitive calcium and potassium channels on the cell body, dendrites,
and initial segment, which act on a much slower time scale and modu-late the
rate at which the neuron discharges. For example, some types of potassium
channels opened by depolarization of the cell result in slowing of further
depolarization and act as a brake to limit further action potential discharge.
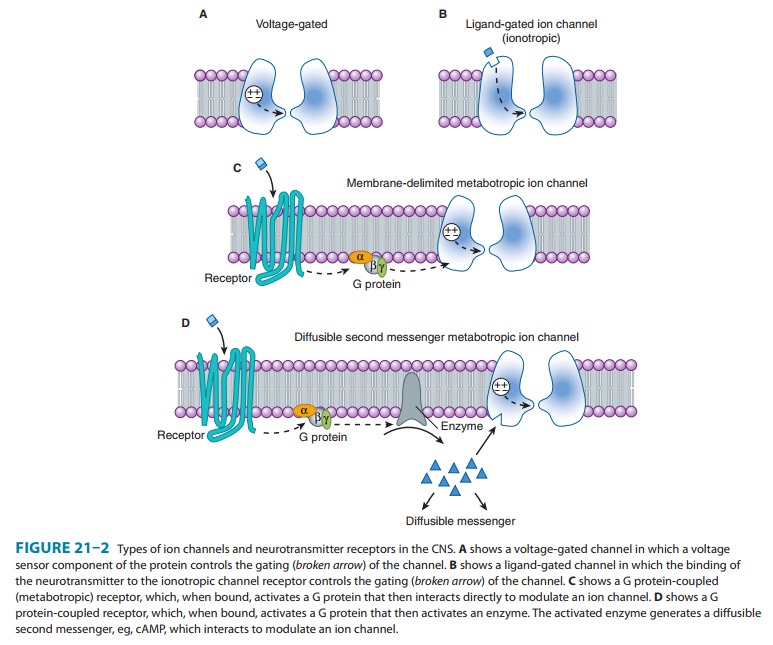
Neurotransmitters exert their effects on neurons by binding to two distinct classes of receptor. The first class is referred to as ligand-gated channels, or ionotropic receptors. The receptorconsists of subunits, and binding of ligand directly opens the channel, which is an integral parts of the receptor complex (see Figure 22–6). These channels are insensitive or only weakly sensi-tive to membrane potential. Activation of these channels typically results in a brief (a few milliseconds to tens of milliseconds) open-ing of the channel. Ligand-gated channels are responsible for fast synaptic transmission typical of hierarchical pathways in the CNS (see following text).
The
second class of neurotransmitter receptor is referred to as metabotropic receptors. These are
seven-transmembrane Gprotein-coupled receptors of the type described earily.
The binding of neurotransmitter to this type of receptor does not result in the
direct gating of a channel. Rather, binding to the receptor engages a G
protein, which results in the production of second mes-sengers that modulate
voltage-gated channels. These interactions can occur entirely with the plane of
the membrane and are referred to as membrane-delimited
pathways (Figure 21–2C). In this case, the G protein (often the βγ subunit) interacts
directly with the voltage-gated ion channel. In general, two types of
voltage-gated ion channels are the targets of this type of signaling: calcium
channels and potassium channels. When G proteins interact with calcium
channels, they inhibit channel function. This mechanism accounts for the
presynaptic inhibition that occurs when presynaptic metabotropic receptors are
activated. In contrast, when these recep-tors are postsynaptic, they activate
(cause the opening of ) potassium channels, resulting in a slow postsynaptic
inhibition. Metabotropic receptors can also modulate voltage-gated channels
less directly by the generation of diffusible
second messengers (Figure 21–2D). A classic example of this type of action
is provided by the β
adrenocep-tor, which generates cAMP via the activation of adenylyl cyclase .
Whereas membrane-delimited actions occur within microdomains in the membrane,
second messenger-mediated effects can occur over considerable distances.
Finally, an important consequence of the involvement of G proteins in receptor
signalling is that, in contrast to the brief effect of ionotropic receptors,
the effects of metabotropic receptor activation can last tens of seconds to
minutes. Metabotropic receptors predominate in the diffuse neu-ronal systems in
the CNS .
Related Topics