Chapter: Clinical Anesthesiology: Clinical Pharmacology: Intravenous Anesthetics
Intravenous Anesthetics: Ketamine
KETAMINE
Mechanisms of Action
Ketamine has multiple effects throughout
the cen-tral nervous system, inhibiting polysynaptic reflexes in the spinal
cord as well as excitatory neurotrans-mitter effects in selected areas of the
brain. In con-trast to the depression of the reticular activating system
induced by the barbiturates, ketamine func-tionally “dissociates” the thalamus
(which relays sensory impulses from the reticular activating sys-tem to the
cerebral cortex) from the limbic cortex (which is involved with the awareness
of sensation). Clinically, this state of dissociative anesthesia may cause the
patient to appear conscious (eg, eye open-ing, swallowing, muscle contracture)
but unable to process or respond to sensory input. Ketamine has been demonstrated
to be an N-methyl-d-aspartate (NMDA)
receptor (a subtype of the glutamate recep-tor) antagonist.
Structure–Activity Relationships
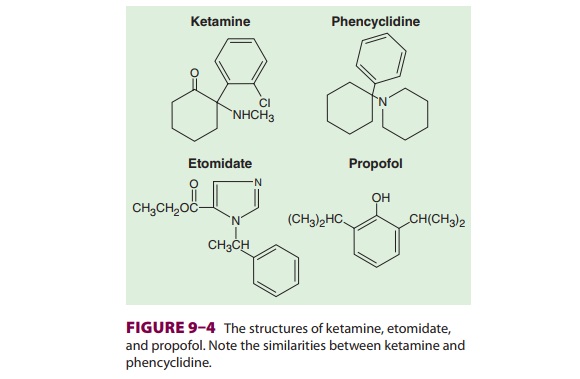
Ketamine (Figure 9–4) is a structural
ana-logue of phencyclidine (an anesthetic that has been used in veterinary medicine,
and a drug of abuse). It is one-tenth as potent, yet retains many
of phencyclidine’s psychotomimetic
effects. Ket-amine is used for intravenous induction of anes-thesia,
particularly in settings where its tendency to produce sympathetic stimulation
are useful (hypovolemia, trauma). When intravenous access is lacking, ketamine
is useful for intramuscular induction of general anesthesia in children and
uncooperative adults. Ketamine can be combined with other agents (eg, propofol
or midazolam) in small bolus doses or infusions for deep conscious sedation
during nerve blocks, endoscopy, etc. Even subanesthetic doses of ketamine may
cause hallu-cinogenic effects but usually do not do so in clinical practice,
where many patients will have received at least a small dose of midazolam (or a
related agent) for amnesia and sedation. The increased anesthetic potency and
decreased psychotomi-metic side effects of one isomer (S[+] versus R[–]) are the result of stereospecific
receptors. The single S(+) stereoisomer
preparation is not available in the United States (but widely available
throughout the world), and it has considerably greater affinity than the
racemic mixture for the NMDA receptor as well as several-fold greater potency
as a general anesthetic.
Pharmacokinetics
A. Absorption
Ketamine has been administered orally,
nasally, rec-tally, subcutaneously, and epidurally, but in usual clinical
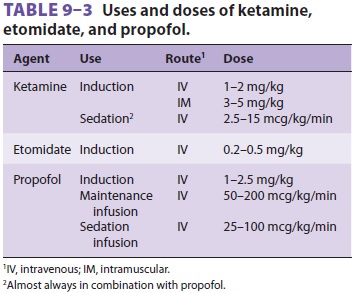
practice it is given intravenously or intra-muscularly (Table 9–3). Peak plasma levels
are usu-ally achieved within 10–15 min after intramuscular injection.
B. Distribution
Ketamine is more lipid soluble and less
protein bound than thiopental. These characteristics, along with
ketamine-induced increase in cere-bral blood flow and cardiac output, lead to
rapid brain uptake and subsequent redistribution (the distribution half-life is
10–15 min). Awakening is due to redistribution from brain to peripheral
compartments.
C. Biotransformation
Ketamine is biotransformed in the liver to several metabolites, one of which (norketamine) retains anesthetic activity. Induction of hepatic enzymes only partially explains the tolerance that patients who receive multiple doses of ketamine will develop. Extensive hepatic uptake (hepatic extraction ratio of 0.9) explains ketamine’s relatively short elimination half-life (2 h).
D. Excretion
End products of ketamine
biotransformation are excreted renally.
Effects on Organ Systems
A. Cardiovascular
In contrast to other anesthetic agents,
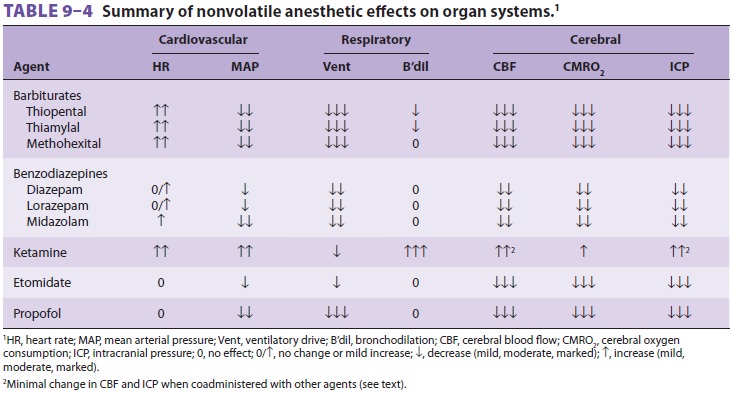
ket-amine increases arterial blood pressure, heartrate, and cardiac output (Table 9–4),
particularly after rapid bolus injections. These indirect cardio-vascular
effects are due to central stimulation of the sympathetic nervous system and
inhibition of the reuptake of norepinephrine after release at nerve terminals.
Accompanying these changes are increases in pulmonary artery pressure and
myocardial work. For these reasons, large bolus injections of ketamine should
be administered cautiously in patients with coronary artery dis-ease,
uncontrolled hypertension, congestive heart failure, or arterial aneurysms. The
direct myocar-dial depressant effects
of large doses of ketamine,probably due to inhibition of calcium transients,
are unmasked by sympathetic blockade (eg, spinal cord transection) or
exhaustion of catecholamine stores (eg, severe end-stage shock). On the other
hand, ketamine’s indirect stimulatory
effects may be beneficial to patients with acute shock.
B. Respiratory
Ventilatory drive is minimally affected by induction doses of ketamine, although rapid intravenous bolus administration or combinations of ketamine with opioids occasionally produce apnea. Racemic ket-amine is a potent bronchodilator, making it a good induction agent for asthmatic patients; however, S(+) ketamine produces minimal bronchodilation. Upper airway reflexes remain largely intact, but par-tial airway obstruction may occur, and patients at increased risk for aspiration pneumonia (“full stom-achs”) should be intubated during ketamine general anesthesia. The increased salivation associated with ketamine can be attenuated by premedication with an anticholinergic agent such as glycopyrrolate
C. Cerebral
The received dogma about ketamine is
that it increases cerebral oxygen consumption, cerebral blood flow, and
intracranial pressure. These effects would seem to preclude its use in patients
with space-occupying intracranial lesions such as occur with head trauma;
however, recent publications offer convincing evidence that when combined with
a benzodiazepine (or another agent acting on the same GABA receptor system) and
controlled ventilation, but not with nitrous oxide, ketamine is not associated with increased
intracranial pres-sure. Myoclonic activity is associated with increased
subcortical electrical activity, which is not apparent on surface EEG.
Undesirable psychotomimetic side effects (eg, disturbing dreams and delirium)
dur-ing emergence and recovery are less common in children and in patients
premedicated with benzo-diazepines or those in whom ketamine is combined with
propofol in a TIVA technique. Of the nonvola-tile agents, ketamine comes
closest to being a “com-plete” anesthetic as it induces analgesia, amnesia, and
unconsciousness.
Drug Interactions
Ketamine interacts synergistically (more
than addi-tive) with volatile anesthetics but in an additive way with propofol,
benzodiazepines, and other GABA-receptor–mediated agents. In animal
experi-ments nondepolarizing neuromuscular blocking agents are minimally
potentiated by ketamine . Diazepam and midazolam attenuate ketamine’s
cardiostimulatory effects and diazepam prolongs ketamine’s elimination
half-life. α-Adrenergic and
β-adrenergic
antagonists(and other agents and techniques that diminish sympathetic
stimulation) unmask the direct myo-cardial depressant effects of ketamine,
which are normally overwhelmed by sympathetic stimulation. Concurrent infusion
of ketamine and propofol, often in a fixed infusion rate ratio of 1:10, has
achieved great popularity for sedation with local and regional anesthesia,
particularly in office-based settings.
Related Topics