Chapter: Clinical Anesthesiology: Clinical Pharmacology: Intravenous Anesthetics
Intravenous Anesthetics: Barbiturates
Intravenous Anesthetics
General anesthesia began with inhaled
agents but now can be induced and maintained with drugs that enter the patient
through a wide range of routes. Drug administration can be oral, rectal,
transder-mal, transmucosal, intramuscular, or intravenous for the purpose of
producing or enhancing an anes-thetic state. Preoperative sedation of adults is
usu-ally accomplished by way of oral or intravenous routes. Induction of
general anesthesia in adults usually includes intravenous drug administration.
Effective topical anesthesia with EMLA (eutectic mixture of local anesthetic)
cream, LMX (plain lidocaine cream 4% and 5%), or 2% lidocaine jelly has
increased the ease of intravenous inductions in children. Maintenance of
general anesthesia is feasible with a total intravenous anesthesia (TIVA)
technique.
BARBITURATES
Mechanisms of Action
Barbiturates depress the reticular activating sys-tem in the brainstem, which controls multiple vital functions, including consciousness. In clinical con-centrations, barbiturates more potently affect the function of nerve synapses than axons. Their primary mechanism of action is believed to be through bind-ing to the γ-aminobutyric acid type A (GABAA) receptor. Barbiturates potentiate the action of GABA in increasing the duration of openings of a chloride-specific ion channel.
Structure–Activity Relationships
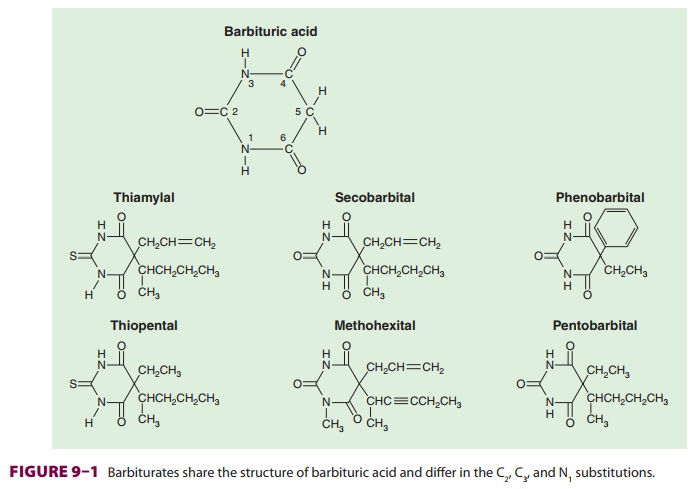
Barbiturates are derived from barbituric
acid (Figure
9–1). Substitution at carbon C5
determines hypnotic potency and anticonvulsant activity. A long-branched chain
conveys more potency than does a short straight chain. Likewise, the phenyl
group in phenobarbital is
anticonvulsive, whereas the methylgroup in methohexital
is not. Replacing the oxygen at C2 (oxybarbiturates)
with a sulfur atom (thio-barbiturates)
increases lipid solubility. As a result, thiopental and thiamylal have a
greater potency, more rapid onset of action, and shorter durations of action
(after a single “sleep dose”) than pentobar-bital. The sodium salts of the
barbiturates are water soluble but markedly alkaline (pH of 2.5% thiopen-tal >10)
and relatively unstable (2-week shelf-life for
2.5% thiopental solution). Concentrations greater than recommended cause
an unacceptable incidence of pain on injection and venous thrombosis.
Pharmacokinetics
A. Absorption
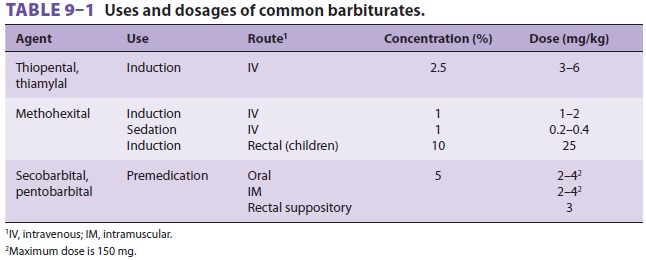
In clinical anesthesiology, thiopental,
thiamylal, and methohexital were frequently administered intrave-nously for
induction of general anesthesia in adults and children (prior to the
introduction of propofol). Rectal thiopental or, more often, methohexital has
been used for induction in children, and intramus-cular (or oral) pentobarbital
was often used in the past for premedication of all age groups.
B. Distribution
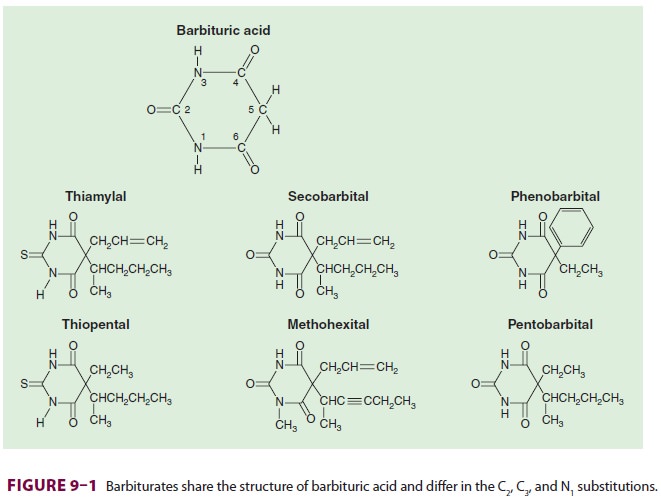
The duration of sleep doses of the
highly lipid-solu-ble barbiturates (thiopental, thiamylal, and metho-hexital)
is determined by redistribution, not by metabolism or elimination. For example,
although thiopental is highly protein bound (80%), its great lipid solubility
and high nonionized fraction (60%)
account for rapid brain uptake (within
30 s). If the central compartment is contracted (eg, hypovolemic shock), if the
serum albumin is low (eg, severe liver disease or malnutrition), or if the
nonionized frac-tion is increased (eg, acidosis), larger brain and heart
concentrations will be achieved for a given dose. Redistribution to the
peripheral compartment— specifically, the muscle group—lowers plasma and brain
concentration to 10% of peak levels within 20–30 min (Figure 9–2). This
pharmacokinetic profile correlates with clinical experience—patients typically
lose consciousness within 30 s and awaken within 20 min.
The minimal induction dose of thiopental
will depend on body weight and age. Reduced induction doses are required for
elderly patients primarily due to slower redistribution. In contrast to the
rapid initial distribution half-life of a few minutes, elimination of
thiopental is prolonged (elimination half-life ranges of 10–12 h). Thiamylal
and methohexital have similar distribution patterns, whereas less lipid-soluble
barbi-turates have much longer distribution half-lives and durations of action
after a sleep dose. Repetitive administration of barbiturates (eg, infusion of
thiopental for “barbiturate coma” and brain protection) saturates the
peripheral compartments,minimizing any effect of redistribution, and render-ing
the duration of action more dependent on elimi-nation. This is an example of
context sensitivity.
C. Biotransformation
Barbiturates are principally
biotransformed via hepatic oxidation to inactive water-soluble metabo-lites.
Because of greater hepatic extraction, metho-hexital is cleared by the liver
more rapidly than thiopental. Although redistribution is responsible for the
awakening from a single sleep dose of any of these lipid-soluble barbiturates,
full recovery of psy-chomotor function is more rapid following metho-hexital
due to its enhanced metabolism.
D. Excretion
Increased protein binding decreases
barbiturate glo-merular filtration, whereas increased lipid solubility tends to
increase renal tubular reabsorption. Except for the less protein-bound and less
lipid-soluble agents such as phenobarbital, renal excretion is lim-ited to
water-soluble end products of hepatic bio-transformation. Methohexital is
excreted in the feces.
Effects on Organ Systems
A. Cardiovascular
Intravenous bolus induction doses of
barbiturates cause a decrease in blood pressure and an increase in heart rate.
Hemodynamic responses to barbiturates are reduced by slower rates of induction.
Depression of the medullary vasomotor center produces vaso-dilation of
peripheral capacitance vessels, which increases peripheral pooling of blood,
mimicking a reduced blood volume. Tachycardia following administration is
probably due to a central vagolytic effect and reflex responses to decreases in
blood pressure. Cardiac output is often maintained by an increased heart rate
and increased myocardial con-tractility from compensatory baroreceptor
reflexes. Sympathetically induced vasoconstriction of resis-tance vessels
(particularly with intubation under light planes of general anesthesia) may actually
increase peripheral vascular resistance. However, in situations where the
baroreceptor response will be blunted or absent (eg, hypovolemia, congestive
heart failure, β-adrenergic blockade), cardiac output and arterial blood
pressure may fall dramatically due to uncompensated peripheral pooling of blood
and direct myocardial depression. Patients with poorly controlled hypertension
are particularly prone to wide swings in blood pressure during anesthesia
induction. The cardiovascular effects of barbiturates therefore vary markedly,
depending on rate of administration, dose, volume status, baseline autonomic
tone, and preexisting cardiovascular dis-ease. A slow rate of injection and
adequate preopera-tive hydration attenuates or eliminates these changes in most
patients.
B. Respiratory
Barbiturates depress the medullary
ventilatory cen-ter, decreasing the ventilatory response to hypercap-nia and
hypoxia. Deep barbiturate sedation often leads to upper airway obstruction;
apnea often fol-lows an induction dose. During awakening, tidal volume and
respiratory rate are decreased follow-ing barbiturate induction. Barbiturates
incompletely depress airway reflex responses to laryngoscopy and intubation,
and airway instrumentation may lead to bronchospasm (in asthmatic patients) or
laryngo-spasm in lightly anesthetized patients.
C. Cerebral
Barbiturates constrict the cerebral
vascula-ture, causing a decrease in cerebral bloodflow, cerebral blood volume,
and intracranial pres-sure. Intracranial pressure decreases to a greater extent
than arterial blood pressure, so cerebral perfusion pressure (CPP) usually
increases. (CPP equals cerebral artery pressure minus the greater of jugular
venous pressure or intracranial pressure.) Barbiturates induce a greater
decline in cerebral oxygen consumption (up to 50% of normal) than in cerebral
blood flow; therefore the decline in cerebral blood flow is not detrimental.
Barbiturate-induced reductions in oxygen requirements and cerebral metabolic
activity are mirrored by changes in the electroencephalogram (EEG), which
progress from low-voltage fast activity with small doses to high-voltage slow
activity, burst suppression, and electrical silence with larger doses.
Barbiturates may protect the brain from transient episodes of focal ischemia
(eg, cerebral embolism) but probably do not protect from global ischemia (eg,
cardiac arrest). Abundant animal data document these effects but the clinical
data are sparse and inconsistent. Furthermore, thio-pental doses required to
maintain EEG suppression (most often burst suppression or flat line) are
associ-ated with prolonged awakening, delayed extubation, and the need for
inotropic support.
The degree of central nervous system
depres-sion induced by barbiturates ranges from mild seda-tion to unconsciousness,
depending on the dose administered (Table 9–1). Some patients relate a taste
sensation of garlic, onions, or pizza dur-ing induction with thiopental.
Barbiturates do not impair the perception of pain. In fact, they some-times
appear to lower the pain threshold. Small doses occasionally cause a state of
excitement and disorientation that can be disconcerting when seda-tion is the
objective. Barbiturates do not produce
muscle relaxation, and some induce
involuntary skeletal muscle contractions (eg, methohexital). Relatively small
doses of thiopental (50–100 mg intravenously) rapidly (but temporarily) control
most grand mal seizures. Unfortunately, acute toler-ance and physiological
dependence on the sedative effect of barbiturates develop quickly.
D. Renal
Barbiturates reduce renal blood flow and
glomeru-lar filtration rate in proportion to the fall in blood pressure.
E. Hepatic
Hepatic blood flow is decreased. Chronic
expo-sure to barbiturates has opposing effects on drug biotransformation.
Induction of hepatic enzymes increases the rate of metabolism of some drugs,
whereas binding of barbiturates to the cytochrome P-450 enzyme system
interferes with the biotrans-formation of other drugs (eg, tricyclic
antidepres-sants). Barbiturates promote aminolevulinic acid synthetase, which
stimulates the formation of porphyrin (an
intermediary in heme synthesis).This may precipitate acute intermittent
porphyria or variegate porphyria in susceptible individuals.
F. Immunological
Anaphylactic or anaphylactoid allergic
reactions are rare. Sulfur-containing thiobarbiturates evoke mast cell
histamine release in vitro, whereas oxyba-rbiturates do not. For this reason,
some anesthesiol-ogists prefer induction agents other than thiopental or
thiamylal in asthmatic or atopic patients, but the evidence for this choice is
sparse. There is no question that airway instrumentation with light anesthesia
is troublesome in patients with reactive airways.
Drug Interactions
Contrast media, sulfonamides, and other
drugs that occupy the same protein-binding sites as thiopental may displace the
barbiturate, increasing the amount of free drug available and potentiating the
organ sys-tem effects of a given dose. Ethanol, opioids, antihistamines, and
other central nervous system depressants potentiate the sedative effects of
barbiturates. The common clinical impression that chronic alcohol abuse is
associ-ated with increased thiopental requirements during induction lacks
scientific proof.
Related Topics