Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Interferons and Interleukins
Interleukins: Nomenclature and Functions
INTERLEUKINS: NOMENCLATURE AND FUNCTIONS
ILs are primarily a collection of immune cell growth, differentiation
and maturation factors. Collectively they orchestrate a precise and efficient
immune response to toxins and pathogens, including cancer cells, recognized as
foreign. As is the case for IFNs, ILs bind to related specific cell surface
receptors which activate similar intracellular signaling cascades (Lutfalla et
al., 2003; Pestka et al., 2004b; Huising et al., 2006). Many ILs, primarily
those with proin-flammatory function, are intrinsically toxic either directly
or indirectly, i.e., through induction of toxic gene products. Therefore the
human body has an elaborate system of checks and balances that, under
(patho)-physiological conditions, regulates the mag-nitude and duration of an
immune response. Under biological conditions, ILs usually have a short
circulation time, and their production is regulated by positive and negative
feedback loops. Furthermore their effect is mostly localized, and in some cases
soluble receptors (sR) or neutralizing antibodies limit their dissemination.
Specific receptor antagonists can also control their activity.
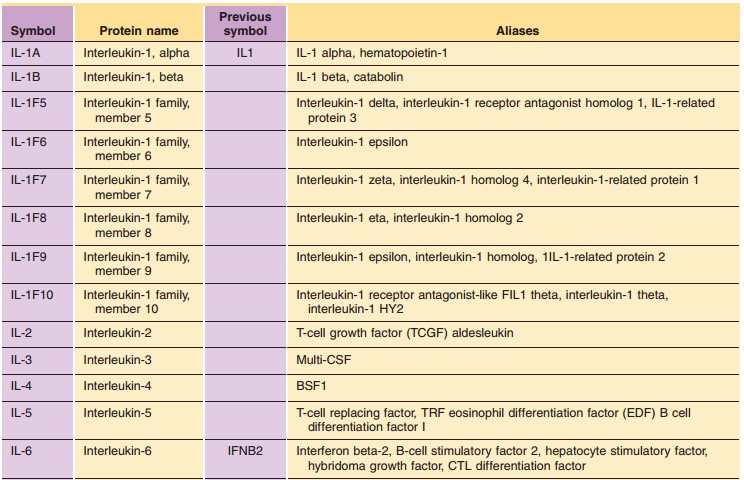
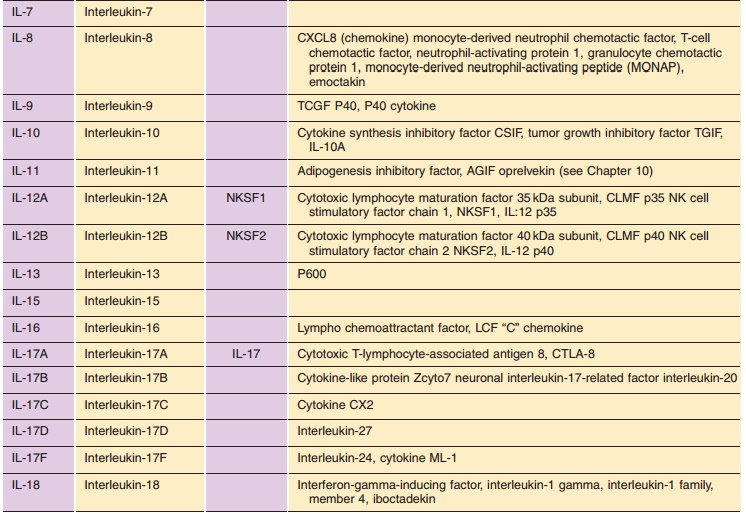
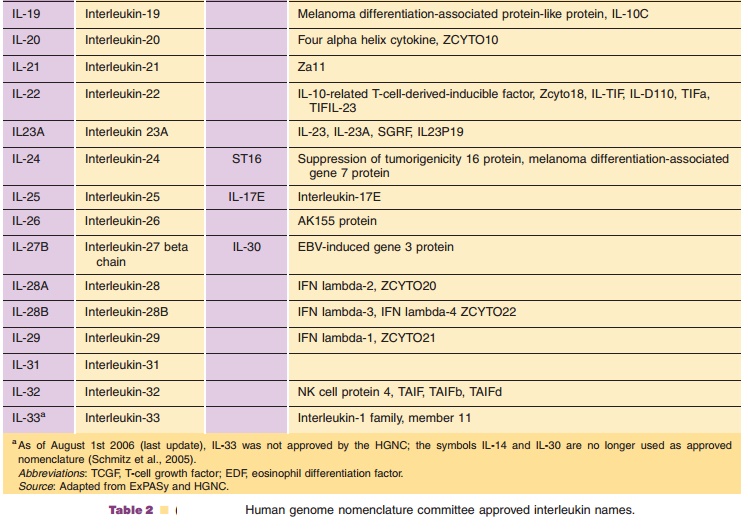
Table 2 lists the ILs for which the protein and gene structure have been
characterized. Their names and symbols have been approved by the HGNC.
Under physiological conditions, the relative concentrations of agonistic
and antagonistic ILs establish a delicate balance in driving pro- and
anti-inflammatory processes. This balance can be dis-turbed by various
pathogenic agents or mechanisms:
· Infectious agents or toxins
· Allergens
· Malignant tumors
· Genetic variants
These pathogenic agents or mechanisms result in a self-limited or
protracted disequilibrium. Symptoms of disease can be the consequence of an
adequate immune response at the end of which the steady-state is reestablished.
A brisk inflamma-tory response is the sign of a healthy immune reaction. In
some instances an inadequate response can manifest itself as relapsing
remitting progres-sive disease, e.g. rheumatoid arthritis, asthma, chronic
inflammatory bowel disease, multiple sclerosis, chronic hepatitis, or chronic
insulitis leading to diabetes mellitus. All have in common that they need a
genetic predisposition and an environmental trigger factor to become active,
and are at best only partially understood. In many cases these diseases are
caused by either insufficient production or overproduction of key ILs. Thus, in
principle, once the diagnosis is made these ILs can be therapeutically
supplemented or suppressed to restore proper balance (Ryff, 1996).
Our current knowledge of the ILs listed in Table 2 is briefly summarized
below and each reference selected expands on the subject. Readers interested in
the current knowledge about the protein, DNA, RNA, gene, chromosome location,
etc. for individual IFNs or ILs is referred to the following databases:
1.
www.genatlas.org (with a links to
other data-bases), or
2.
http://au.expasy.org/sprot/, or
3.
www.rcsb.org/pdb/ for the 3D
models of indivi-dual IFNs or ILs.
Interleukin-1 Family
IL-1 (Towne et al., 2004) is generally used to describe IL-1a and IL-1b both of which have the same biological effects and play a primordial
role in the innate and adaptive immune response. Although the prototypical
proinflammatory cytokine, it also plays a key role in hematopoiesis, appetite
control, and bone metabolism.
IL-1 is released as part of the acute phase reaction of hepatocytes. The
primary producers of IL-1 are macrophages, B-cells and neutrophils. IL-1a and IL-1b are synthesized as propeptides of approxi-mately 30 kDa, and are then
cleaved to produce products of 159 and 153 amino acids. Differences in
glycosylation are responsible for the wide variation of reported molecular
weights.
There are several other members of the IL-1 family. One is a naturally
occurring inhibitor of IL-1. This IL-1 receptor antagonist (IL-1Ra) has limited
sequence similarity to either IL-1a or IL-1b, but does have the ability to bind to the IL-1 receptors. Lacking IL-1
activity, it acts as a useful blocker of the receptor. A recombinant IL-1Ra has
been investigated for its potential use in sepsis; however, the clinical trials
were inconclusive. Recombinant IL-1Ra has however been used successfully in
treatment of rheumatoid arthritis and is marketed under the name of Kineret
(see section “Therapeutic Use of Recombinant Interleukins”).
Interleukin 2
IL-2 (Nelson, 2004) is a typical four a helix cytokine originally described as T-cell GF (TCGF). Synthesized and secreted primarily by T-cells, IL-2 can stimulate the growth, differentiation and activation of T-cells, B-cells, and NK-cells. The new understanding of IL-2 biology is that its major physiological effect is to promote self-tolerance by suppressing T-cell response in vivo. Three chains comprise the cellular high affinity IL-2 receptor—the α, β and γ chains. IL-15 is closely related to IL-2 with which it shares the β and γ signaling receptor subunits. A soluble form of the IL-2R capable of binding IL-2, a truncated version of the a chain without cytoplasmic tail, has been found in human serum (sR). High levels of IL-2sR have been found in patients with a wide variety of disorders, including chronic hepatitis C, HIV infection, cancer, solid organ transplant rejection, and arthritis. Soluble IL-2R can bind released IL-2 prior to its binding to cells to preventoverflow or over-stimulation. Several other cytokine and adhesion molecule receptors also have circulat-ing forms. This is one manner in which the immunological cascade maintains its checks and balances.
Interleukin 3
generation of hematopoietic progenitors of every lineage. Administration
of IL-3 produces an increase in erythrocytes, neutrophils, eosinophils,
monocytes and platelets. IL-3, however, is not involved inconstitutive
hematopoiesis but rather in inductive hematopoiesis upon exposure to
immunological stress. IL-3 can act synergistically or additively with other
hematopoietic GFs.
Interleukin 4
IL-4 (Steinke and Borish, 2001) is produced by Th2 cells and by mast
cells, basophils, and eosinophils. It stimulates B-cell proliferation and
activation, induces class switch to IgE and IgG1 expression from B-cells, as well
as class II Major Histocompatibility Complex (MHC) expression. In addition it
induces the differentiation of eosinophils and activity of cytotoxic T-cells.
IL-4 regulates the differentiation of helper T-cells to the Th2 type. These
T-cells produce the cytokines IL-4, IL-5, IL-9, and IL-13, which can all
participate in the allergic response. IL-4 regulates the production of IgE by
B-lymphocytes. It also has the ability to stimulate CK production and mucus
hypersecretion by epithelial cells. IL-4 can elicit many responses, some of
which are associated with allergy and asthma.
Interleukin 5
IL-5 (Greenfeder et al., 2001) acts as a homodimer originally known as
T-cell replacement factor (TRF), eosinophil differentiation factor (EDF) and
B-cell GF (BCGFII). It is produced by a number of cell types. It acts on the
eosinophilic lineage, stimulating eosino-phil expansion and chemotaxis and also
has activity on basophils. In humans IL-5 is a very selective cytokine as only
eosinophils and basophils express IL-5 receptors.
Interleukin 6
IL-6 (Heinrich et al., 1990) is produced by lymphoid and nonlymphoid
cells. By stimulating hepatocytes to produce “acute phase proteins” it plays a
central role in the “acute phase reaction.” It is also responsible for the
reactive thrombocytosis seen in acute inflamma-tory processes by stimulating
thrombopoietin (TPO) (Kaushansky, 2005). IL-6 was formerly known as IFNβ2 for its weak antiviral activity.
IL-6 is also associated with insulin resistance in obese individuals (Esposito
et al., 2003).
Interleukin 7
IL-7 (Fry and Mackall, 2002) is a glycosylated essen-tially
tissue-derived cytokine. Its primary sources are stromal and epithelial cells
in various locations includ-ing intestinal epithelium, liver and to a lesser
degree dendritic cells. IL-7 acts primarily on pre-B-cells to stimulate their
differentiation. It can also stimulate the development of human T-cells. IL-7
is classified as a type I short chain cytokine of the hematopoietin family
which also includes IL-2, IL-3, IL-4, IL-5, GM-CSF, IL-9, IL-13, IL-15, M-CSF
and stem cell factor (SCF).
Interleukin 8
IL-8 (Remick, 2005) is a 6 to 8 kDa CXC CK, a potent chemoattractant for
neutrophils. It affects the proin-flammatory effector side, including the
stimulation of neutrophil degranulation and the enhancement of neutrophil
adherence to endothelial cells. It is produced by monocytes, macrophages, fibroblasts,
keratinocytes and endothelial cells. Elevated levels of IL-8 have been found in
psoriatic arthritis, synovial fluid and syno-vium. IL-8 has been implicated in
angiogenesis.
Interleukin 9
IL-9 (Zhou et al., 2001) is a Th2 cytokine originally characterized as a
factor produced by activated T-cells and able to support the long-term growth
of some T-helper clones. IL-9 activities extend to various cell types including
mast cells, B-lymphocytes, hemo-poietic progenitors, eosinophils, lung
epithelial cells,neuronal precursors and T-lymphocytes. Increased IL-9
production has been implicated in major pathologies such as asthma supported by
its effects on IgE production, mucus production, mast cell differentia-tion,
eosinophil activation and bronchial hyperre-sponsiveness. IL-9 stimulates the
growth of murine thymic lymphomas and an autocrine loop has been suggested in
Hodgkin lymphoma. Finally, IL-9 is required for an efficient immune response
against intestinal parasites. IL-9 exerts its effects through a receptor that
belongs to the hemopoietic receptor superfamily and consists of two chains,
also involved in IL-2, IL-4, IL-7, and IL-15 signaling.
Interleukin 10
Macrophages are the major source of IL-10 (Asadullah, 2004), which Th2
cell subsets, monocytes and several other cells can also synthesize. It is a
homodimer, whereby each monomer consists of 160 amino acids with a MW of 18.5
kDa. IL-10 is a major endogenous anti-inflammatory mediator which acts by
profoundly inhibiting the synthesis of proinflam-matory molecules. A number of
molecules produced under stress conditions including reactive oxygen species
stimulate IL-10 synthesis. Recombinant hu-man IL-10 has been tested in clinical
trials in rheumatoid arthritis, inflammatory bowel disease, psoriasis, organ
transplantation, and chronic hepatitis C. The results are mixed, however they
give new insight into the immunobiology of IL-10 and suggest that the
IL-10/IL-10R system may become a new therapeutic target. Several novel IL-10
related class 2 cytokines have recently been discovered (Pestka et al., 2004b).
These include IL-19, -20, -22, -24, -26, -28A, -28B and -29. The receptor
complexes for IL-22, IL-26, IL-28A, IL-28B and IL-29 are distinct from that
used by IL-10; however, all of these cytokines use a common second chain, IL-10
receptor-2 (IL-10R2; CRF2-4), to assemble their active receptor complexes
(Donnelly et al. 2004; Pestka et al., 2004b).
Interleukin 11
IL-11 (Du and Williams, 1997) initially described as hematopoietic
factor with thrombopoietic activity has subsequently been shown to be expressed
and active in many other tissues including brain, spinal cord neurons, gut, and
testes. IL-11 acts synergistically with other cytokines such as IL-3, -4, -7,
-12, -13, SCF and GM-CSF to stimulate various stages and lineages of
hematopoiesis and in particular with IL-3 and TPO, also termed megakaryocyte
growth and development factor (MGDF), to stimulate various stages of
mega-karyocytopoiesis and thrombopoiesis. Treatment with IL-11 results in production,
differentiation, andmaturation of megakaryocytes. IL-11 also has a direct
effect on erythroid progenitors and also modulates the differentiation and
maturation of myeloid progenitor cells. Alveolar and bronchial epithelial cells
produce IL-11, which is upregulated by inflammatory cyto-kines and respiratory
syncitial virus (RSV) suggesting that it plays a role in pulmonary
inflammation. IL-11 also is an important regulator of bone metabolism.
Interleukin 12
IL-12 (Adorini, 1999) is a 75-kDa heterodimeric proinflammatory cytokine
composed of two cova-lently linked glycosylated chains: p35 and p40. It is
produced predominantly by activated monocytes and dendritic cells, enhances
proliferation and cytolytic activity of NK- and T-cells, and stimulates their IFNγ
production, guiding them toward a Th1 response while it inhibits Th2 cells.
Because of these properties, the regulation of IL-12 production and its
alteration in certain disease states have major relevance for the modulation of
immune and allergic responses, and recombinant IL-12 has several potential
therapeutic uses in infectious diseases, allergy, and cancer.
Interleukin 13
Human IL-13 (Wills-Karp, 2004) is a 17 kDa glycopro-tein cloned from
activated T-cells. IL-13 was first recognized for its effects on B cells and
monocytes, where it upregulated class II expression, promoted IgE class
switching and inhibited inflammatory cytokine production. IL-13 possesses
several unique effector functions that distinguish it from IL-4. Resistance to most
gastrointestinal nematodes is mediated by type-2 cytokine responses, in which
IL-13 plays a dominant role. By regulating cell-mediated immunity, IL-13
modulates resistance to intracellular organisms. In the lung, IL-13 is the
central mediator of allergic asthma, where it regulates eosinophilic
inflammation, mucus secretion, and airway hyperresponsiveness. IL-13 can also
inhibit tumor immunosurveillance. Thus, inhibi-tors of IL-13 might be effective
as cancer immunother-apeutics by boosting type-1-associated antitumor defenses.
Investigations into the mechanisms that regulate IL-13 production and/or
function have shown that IL-4, IL-9, IL-10, IL-12, IL-18, IL-25, IFNγ, TGF-b, TNF-a, and the IL-4/IL-13 receptor complex play important roles in this
process.
Interleukin 15
IL-15 (Fehniger and Caligiuri, 2001) shares the IL-2bg receptor complex components IL-2Rb (CD122)
and IL-2Rg (CD132), however specificity is conferred by a unique a-chain (IL-15Ra) completing the IL-15Rabg heterotrimeric high affinity receptor complex. IL-15exhibits
characteristics of IL-2 such as stimulation of T-cells, but the third receptor
chain (IL-15Ra) distin-guishes its activities and specificities. In addition, IL-15
and its receptor have a much wider tissue distribution than IL-2 and its
receptor.
Interleukin 16
IL-16 (Cruikshank et al., 1998) is a proinflammatory cytokine, which
induces chemotaxis of CD4þ T-cells, monocytes and
eosinophils. It has been shown to play a role in asthma (El Bassam et al.,
2005), Crohn’s Disease (CD) (Middel et al., 2001) and systemic lupus
erythematosus (SLE) (Lee et al., 1998). IL-16 also inhibits human (HIV) and
simian (SIV) immunodefi-ciency virus. A neuronal form of IL-16 detected in
neurons of the cerebellum and hippocampus has been described (Kurschner and
Yuzaki, 1999).
Interleukin 17 Family
IL-17 (Kolls and Linde´, 2004) a homodimeric glycoprotein more recently
renamed IL-17A is the prototypic IL-17 family member. It is produced by
activated T-cells, but its receptor is expressed in every tissue examined.
Subsequently, five additional family members IL-17B to IL-17F have been
discovered. The importance of this family of cytokines and their receptors
expressed in disparate tissues goes beyond the modulation of T-cell-mediated
inflammatory response and importance in effective host defense against
gram-negative bacteria. IL-17 cytokines have also a role in the homeostasis of
tissues and the progression of diseases such as arthritis (Gaffen, 2004;
Lubberts et al., 2005), and cancer, e.g. prostate cancer (Moseley et al.,
2003).
Interleukin 18
IL-18 (Liu et al., 2000) shares unique structural features with the IL-1
family, but it does not have the usual four-helix structure rather an all β-pleated
sheet structure. It is produced by activated macro-phages such as Kupffer cells
of the liver and other resident macrophages from which the mature protein is
released. IL-18 is an early inducer of the Th1 response, costimulating, with
IL-12, the production of IFNγ, TNF-a, GM-CSF
and IL-2. IL-18 is associated with the Metabolic Syndrome and coronary vascular
disease (Hung et al., 2005).
Interleukin 19
IL-19 (Gallagher et al., 2000; Chang et al., 2003) is a newly discovered
member of the IL-10 family whose function is presently undefined. The induction
of IL-19 in human monocytes is downregulated by IFNγ
and upregulated by IL-4. IL-19 plays a role in the Th1/Th2 system. IL-19
is able to influence the maturation of human T-cells. CD4þ T-cells resulting from staphylococcal enterotoxin B (SEB) stimulation
in the presence of IL-19 contained a higher proportion of IL-4 producing cells
than those developing in the absence of IL-19. This observation was
complemented by the observation that fewer IFNγ cells accrued in the presence
of IL-19, thereby suggesting that IL-19 altered the balance of Th1/Th2 cells in
favor of Th2 cells. Furthermore, in cultures of whole peripheral blood
mononuclear cells (PBMC) IL-19 upregulated IL-4 and downregulated IFNγ in a
dose-dependent manner.
Interleukin 20
IL-20 (Blumberg et al., 2001) was originally identified from a
keratinocyte library, mRNA isolated from skin and trachea. Analysis of the
entire coding sequence yielded a 176 amino acid sequence classified as a
helical cytokine member of the IL-10 family. IL-1b, TGF-a and epidermal GF (EGF), factors known to be involved with proliferative
and proinflammatory signals in the skin, enhance the response to IL-20. Two
orphan class 2 cytokine receptors, both expressed in skin, have been discovered
and labeled IL-20Ra and IL-20Rb; both are required for IL-20 binding and mRNA for both are markedly
upregulated in psoriatic compared to normal skin.
Interleukin 21
IL-21 (Sivakumar et al., 2004), a four-helix cytokine, is a new member
of the IL-2 family of cytokines that utilize the common g-chain receptor subunit for signal transduction. The heterodimeric
IL-21R has a IL-21-specific subunit besides the g-chain.
IL-21 expression is restricted primarily to activated CD4þ T-cells. IL-21 expression seems transient and stage specific during
T-cell differentiation. It is required for normal humor-al immunity and
regulates antibody production in cooperation with IL-4. IL-21 also regulates
cell-mediated immunity by inducing IFNγ, TNF-a, synth-esis
of perforin and granzyme B leading to cytolytic activity. It can cooperate with
other cytokines to generate potent killer T-cells and thus has antitumor
activity. Lastly it also has inhibitory activity by inducing IL-10. Thus,
altogether, it is responsible for the coordination of the initiation and
cessation of an immune response.
Interleukin 22
IL-22 (Kotenko et al., 2001a,b; Boniface et al., 2005) belongs to a
family of cytokines structurally related to IL-10, including IL-19, IL-20,
IL-24, and IL-26. In contrast to IL-10, it has proinflammatory activities: it
upregulates the production of acute phase proteinsand pancreatitis-associated
protein 1. The IL-22 receptor is composed of an IL-22-binding chain, IL-22R1
and the IL-10R2 subunit, which is shared with the IL-10R. IL-22 is produced by
activated human Th cells and mast cells. A soluble IL-22-binding protein,
IL22RBP, encoded by a distinct gene, has been identified. This sR, which has
34% amino acid identity to the extracellular domain of the IL-22R1, binds IL-22
and antagonizes its functional activities. The skin is also a target for IL-22;
high IL-22 expression has been detected in the skin of patients with
T-cell-mediated dermatoses. Normal human epidermal keratinocytes express a
functional receptor for IL-22 but not for IL-10. IL-22 plays a role in skin
inflammatory processes and wound healing.
Interleukin 23
IL-23 (Aggarwal et al., 2003) is a heterodimeric cytokine comprising the
IL-12 p40 subunit and an IL-23-specific p19 subunit. It is produced by
activated dendritic cells and acts on memory CD4þ T-cells.
IL-23 induces IL-17 and thus plays an early role in defense against
gram-negative infection. It is also pivotal for establishing and maintaining
organ-spe-cific inflammatory autoimmune disease. IL-23 and IL-27 both have
potent antitumor activity even against poorly immunogenic tumors using
different effector mechanisms.
Interleukin 24
IL-24 (Jiang et al., 1996; Wang and Liang, 2005) is a novel member of
the IL-10 family secreted by activated PBMC and the ligand for two
heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. The latter is
also a receptor chain for IL-20. Under physiological conditions, the major
sources of IL-24 are activated monocytes and Th2 cells, whereas the major IL-24
target tissues, based on the receptor expression pattern, are nonhematopoietic
in origin, and include skin, lung and reproductive tissues. Structurally and
functionally, IL-24 is highly con-served across species. It has shown
antiangiogenic activity and its gene is a tumor suppressor gene (Ishikawa et
al., 2005).
Interleukin 25
IL-25 (Fort et al., 2001), although structurally related to IL-17, is a
protein of approximately 17 kDa. It is produced by Th2-polarized T-cells, and
its biological effects differ markedly from those of the previously described
IL-17 family members. IL-25 induces IL-4, -5 and -13 and causes histological
changes in the lungs and GI tract, including eosinophilic and mononuclear
infiltrates, increased mucus production, and epithelial cell hyperplasia and
hypertrophy. IL-12 appears to be a key cytokine for the development of
Th2-associatedpathologies such as asthma and other allergic reac-tions, as well
as antiparasitic response.
Interleukin 26
IL-26 (Fickenscher and Pirzer, 2004; Ho¨r, 2004) is a polypeptide of 177
amino acids and is part of the IL-10 family. IL-26 is produced in activated Th1
memory cells, to some extent in NK cells, but neither in Th2 or regulatory
T-cells, nor in monocytes or B-cells. IL-26 is normally expressed by various
sorts of T-cells at low levels, and specifically overexpressed by T-cells after
Herpesvirus
saimiri (HVS) transformation. It binds to
aheterodimeric receptor postulated to be comprised of the IL-20R1 and IL-10R2
chains. Targeting epithelial cells, the T-cell lymphokine IL-26 is likely to
play a role in local mechanisms of mucosal and cutaneous immunity.
Interleukin 27
IL-27 (Villarino and Hunter, 2004) is a novel hetero-dimeric cytokine of
the IL-12 family that consists of EBI3, an IL-12p40-related protein, and p28, a
newly discovered IL-12p35-related polypeptide. It is pro-duced by
antigen-presenting cells and specifically acts on naı¨ve T-cells. IL-27
synergizes with IL-12 to produce IFNγ and does not support Th2 cytokine
production by activated T-cells. Recent evidence however suggests that this
receptor/ligand pair is also required to suppress a variety of immune cell
effector processes, including proliferation and cytokine production. There is
some evidence for the protective role of IL-27 from inflammatory autoimmune
diseases and it has been shown to have antitumor effects in animal models
(Hisada et al., 2004).
Interleukin 28 and Interleukin 29
Recently, the human genomic sequence for a family of three cytokines,
designated IL-28A, IL-28B and IL-29 (Sheppard, 2002), that are distantly
related to type I IFNs and the IL-10 family has been described (Pestka et al.,
2004a,b). Like type I IFNs, IL-28 and IL-29 are induced by viral infection and
have antiviral activity. However, IL-28 and IL-29 interact with a heterodimeric
class 2 cytokine receptor that consists of the IL-10 receptor 2 (IL-10R2) and
an orphan class 2 receptor chain, designated IL-28R1. This newly described
cytokine family may serve as an alternative to type I IFNs in providing
immunity to viral infection.
Interleukin 31
IL-31 (Bilsborough et al., 2006) is a newly discovered four-helix bundle
cytokine preferentially expressed by activated T-cells with a Th2 bias.
Together with IL-4 and IL-13, IL-31 has been implicated in the pathogen-esis of
atopic dermatitis because they are produced by a subset of T-cells that home to
the skin. IL-31 signalsthrough a heterodimeric receptor constitutively
ex-pressed by epithelial cells including keratinocytes. IL-31 stimulated
keratinocytes induce a whole array of inflammatory CKs which also facilitate
the recruit-ment of lymphocytes, monocytes and polymorpho-nuclear cells to the
epidermis.
Interleukin 32
IL-32 (Kim et al., 2005) is a recently characterized polypeptide which
was described several years ago as natural killer cell transcript 4 (NK4) of
activated T-cells and NK-cells and belongs to the proinflamma-tory cytokines.
It induces TNF-a and MIP-2, a CK, in different cells via the signal pathway of
proinflamma-tory cytokines. To date it has been detected in higher
concentration in some of the patients with sepsis compared to healthy
individuals.
Interleukin 33
IL-33 (Schmitz et al., 2005), unlike other members of the IL-1 ligand
family, which are all proinflammatory, has a major role in the development of a
Th2 type immune response by inducing IL-5 and IL-13. Human smooth muscle cells
as well as epithelial cells forming bronchus and small airways show
constitutive expression of IL-33 mRNA; in lung or dermal fibroblasts and keratinocytes
IL-33 mRNA is induced after activation with TNF-a and IL-1b. Activated dendritic cells and macrophages are the only hematopoietic
cells showing low quantities of IL-33 mRNA. In addition, IL-33 and IL-18 are
the only known IL-1 family member genes not located on chromosome 2.
Related Topics