Periodic Classification of Elements - Book Back Questions with Answers | 10th Science : Chapter 8 : Periodic Classification of Elements
Chapter: 10th Science : Chapter 8 : Periodic Classification of Elements
Book Back Questions with Answers
Periodic Classification of Elements
I. Choose the best answer.
1. The number of periods and groups in the periodic table are______.
a) 6,16
b) 7,17
c) 8,18
d) 7,18
2. The basis of modern periodic law is______.
a) atomic number
b) atomic mass
c) isotopic mass
d) number of neutrons
3. _____ group contains the member of halogen family.
a) 17th
b) 15th
c) 18th
d) 16th
4. _____ is a relative periodic property
a) atomic radii
b) ionic radii
c) electron affinity
d) electronegativity
5. Chemical formula of rust is ________.
a) FeO.xH2O
b) FeO4.xH2O
c) Fe2O3.xH2O
d) FeO
6. In the alumino thermic process the role of Al is _____.
a) oxidizing agent
b) reducing agent
c) hydrogenating agent
d) sulphurising agent
7. The process of coating the surface of metal with a thin layer of zinc is called______.
a) painting
b) thinning
c) galvanization
d) electroplating
8. Which of the following have inert gases 2 electrons in the outermost shell.
a) He
b) Ne
c) Ar
d) Kr
9. Neon shows zero electron affinity due to _____.
a) stable arrangement of neutrons
b) stable configuration of electrons
c) reduced size
d) increased density
10. ______ is an important metal to form amalgam.
a) Ag
b) Hg
c) Mg
d) Al
II. Fill in the blanks
1. If the electronegativity difference between two bonded atoms in a molecule is greater than 1.7, the nature of bonding is
2. 6th is the longest period in the periodical table.
3. Atomic number forms the basis of modern periodic table.
4. If the distance between two Cl atoms in Cl2 molecule is 1.98Å, then the radius of Cl atom is 0.99 A.
5. Among the given species A–,A+, and A, the smallest one in size is A+.
6. The scientist who propounded the modern periodic law is Henry Moseley.
7. Across the period, ionic radii decreases (increases,decreases).
8. Lanthanides and Actinides are called inner transition elements.
9. The chief ore of Aluminium is Bauxite.
10. The chemical name of rust is hydrated ferric oxide.
III. Match the following
1. Galvanisation : Noble gas elements
2. Calcination : Coating with Zn
3. Redox reaction : Silver-tin amalgam
4. Dental filling : Alumino thermic process
5. Group 18 elements : Heating in the absence of air
Answer
1. Galvanisation : coating with Zn
2. Calcination : Heating in the absence of air
3. Redox reaction : Alumino thermic process
4. Dental filling : silver-tin amalgam
5. Group 18 elements : Noble gas elements
IV. True or False: (If false give the correct statement)
1. Moseley’s periodic table is based on atomic mass. - False
Moseley's periodic table is based on atomic number.
2. Ionic radius increases across the period from left to right.
Ionic radius decreases across the period from left to right.
3. All ores are minerals; but all minerals cannot be called as ores; - True
4. Al wires are used as electric cables due to their silvery white colour.
A1 wires are used in electric cables as they are good condutors.
5. An alloy is a heterogenous mixture of metals.
An alloy is a homogeneous mixture of metals.
V. Assertion and Reason
Answer the following questions using the data given below:
i. A and R are correct, R explains the A.
ii. A is correct, R is wrong.
iii. A is wrong, R is correct.
iv. A and R are correct, R doesn’t explains A.
1. Assertion : The nature of bond in HF molecule is ionic
Reason : The electronegativity difference between H and F is 1.9
Answer: i. A and R are correct, R explains the A.
2. Assertion : Magnesium is used to protect steel from rusting
Reason : Magnesium is more reactive than iron
Answer: iii. A is wrong, R is correct.
3. Assertion : An uncleaned copper vessel is covered with greenish layer.
Reason : copper is not attacked by alkali
Answer: i. A and R are correct, R explains the A.
VI. Short answer questions
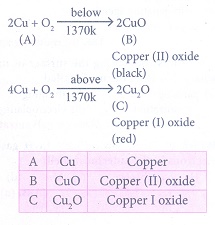
1. A is a reddish brown metal, which combines with O2 at < 1370 K gives B, a black coloured compound. At a temperature > 1370 K, A gives C which is red in colour. Find A,B and C with reaction.
Reddish brown metal A is copper.
2. A is a silvery white metal. A combines with O2 to form B at 800oC, the alloy of A is used in making the aircraft. Find A and B
Silvery white metal is aluminium.

3. What is rust? Give the equation for formation of rust.
When iron is exposed to moist air, it forms a layer of brown hydrated ferric oxide on its surface. This compound is known as rust and the phenomenon of formation of rust is known as rusting.
4Fe + 3O2 + x H20 → 2Fe2O3.xH2O(rust)
4. State two conditions necessary for rusting of iron.
Two conditions necessary for rusting iron are (i) Oxygen (ii) Water.
VII. Long answer questions
1. a) State the reason for addition of caustic alkali to bauxite ore during purification of bauxite.
b) Along with cryolite and alumina, another substance is added to the electrolyte mixture. Name the substance and give one reason for the addition.
Answer:
a) Caustic soda solution at 150° is added to bauxite
ore to obtain Sodium Meta Aluminate.
b) Fluorspar is added to the electrolyte mixture so
as to lower the fusion temperature of the electrolyte.
2. The electronic configuration of metal A is 2,8,18,1.
The metal A when exposed to air and moisture forms B a green layered compound. A with con. H2SO4 forms C and D along with water. D is a gaseous compound. Find A,B,C and D.
Metal A is copper
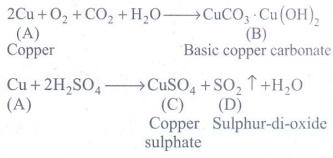
2Cu + O2 + CO2 + H2O → CuCO3.Cu(OH)2
2Cu [A:Copper] + O2 + CO2 + H2O → CuCO3.Cu(OH)2 [B:Basic copper carbonate]
Cu + 2H2SO4 → CuSO4 + SO2 ↑ + H2O
Cu [A:Copper] + 2H2SO4 → CuSO4 [C:Copper sulphate] + SO2 ↑ [D:Sulphur-di-oxide] + H2O
[A: Cu : Copper]
[B: CuCO3.Cu(OH)2 : Basic copper carbonate]
[C: CuSO4 : Copper sulphate]
[D: SO2 : Sulphur-di-oxide]
3. Explain smelting process.
Answer:
Smelting: Smelting is the process of reducing the
roasted metallic oxide from the metal in its molten condition. In this process,
impurities are removed as slag by the addition of flux.
VIII. HOT questions
1. Metal A belongs to period 3 and group 13. A in red hot condition reacts with steam to form B. A with strong alkali forms C. Find A,B and C with reactions
Metal A is Aluminium.
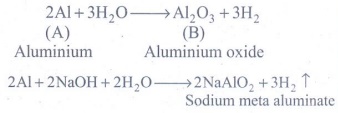
2Al + 3H2O → A12O3 + 3H2
2Al [(A) Aluminium] + 3H2O → A12O3 [(B) Aluminium oxide] + 3H2
2A1 +2NaOH + 2H2O-- >2NaAlO2+3H2 ↑
2A1 +2NaOH + 2H2O-- >2NaAlO2 [C: Sodium meta aluminate] +3H2 ↑
(A) : Al : Aluminium
(B) : A12O3 : Aluminium oxide
(C) : 2NaAlO2 : Sodium meta aluminate
2. Name the acid that renders aluminium passive. Why?
Dilute or concentrated nitric acid does not attack aluminium, but it renders aluminium passive due to the formation of an oxide film on its surface.
3. a) Identify the bond between H and F in HF molecule.
b) What property forms the basis of identification?
c) How does the property vary in periods and in groups?
a) Ionic bond
b) Electronegativity
c) Along the period, from left to right in the periodic table, the electronegativity increases. On moving down a group, the electronegativity of the elements decreases.
Related Topics