Chapter: 10th Science : Chapter 8 : Periodic Classification of Elements
Alloys
ALLOYS
An alloy is a
homogeneous mixture of two or more metals or of one or more metals with certain
non-metallic elements.
The properties of alloys
are often different from those of its components. Pure gold is too soft to be
used. The addition of small percentage of copper enhances its strength and
utility.
1. Amalgam
An amalgam is an alloy
of mercury with another metal. These alloys are formed through metallic bonding
with the electrostatic force of attraction between the electrons and the
positively charged metal ions. Silver tin amalgam is used for dental filling.
Reasons for alloying:
i.
To modify appearance and colour
ii.
To modify chemical activity.
iii.
To lower the melting point.
iv.
To increase hardness and tensile strength.
v.
To increase resistance to electricity.
2. Method of making alloys
·
By fusing the metals together. E.g. Brass is made by melting zinc
and copper.
·
By compressing finely divided metals. E.g. Wood metal: an alloy of
lead, tin, bismuth and cadmium powder is a fusible alloy.
Alloys as solid
solutions:
Alloys can be considered
solid solutions in which the metal with high concentration is solvent and other
metals are solute.
For example, brass is a
solid solution of zinc (solute) in copper (solvent).
3. Types of Alloys
Based on the presence or
absence of Iron, alloys can be classified into:
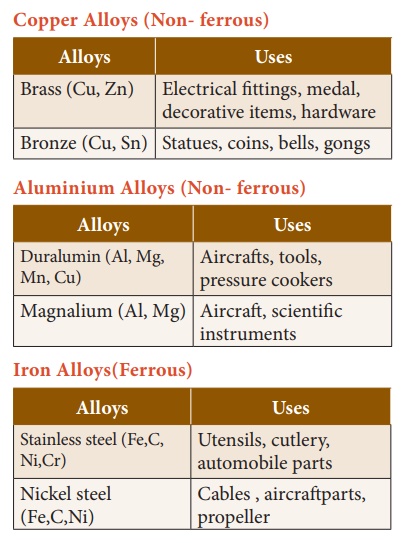
·
Ferrous alloys: Contain Iron as a major component. A few examples
of ferrous alloys are Stainless Steel, Nickel Steel etc.
·
Non-ferrous alloys: These alloys do not contain Iron as a major
component. For example, Aluminium alloy, Copper alloy etc.
Copper Alloys (Non- ferrous), Aluminium Alloys (Non- ferrous), Iron Alloys(Ferrous)
Related Topics