Chapter: Biochemistry: Storage Mechanisms and Control in Carbohydrate Metabolism
How Glycogen Is Produced and Degraded
How Glycogen Is Produced and
Degraded
When we digest a meal high in carbohydrates, we have a supply of
glucose that exceeds our immediate needs. We store glucose as a polymer,
glycogen, that is similar to the starches found in plants; glycogen differs
from starch only in the degree of chain branching. In fact, glycogen is
sometimes called “animal starch” because of this similarity. A look at the
metabolism of glycogen will give us some insights into how glucose can be
stored in this form and made available on demand. In the degradation of
glycogen, several glucose residues can be released simultaneously, one from
each end of a branch, rather than one at a time as would be the case in a
linear polymer.
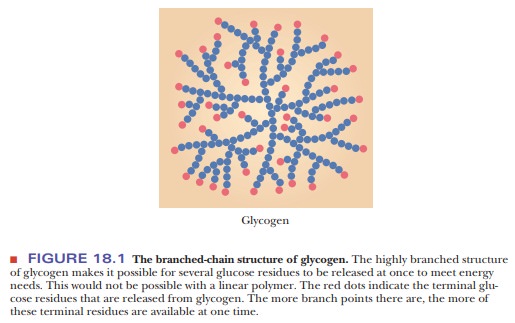
This feature is useful to an organism in meeting short-term demands for energy by increasing the glucose supply as quickly as possible (Figure 18.1). Mathematical modeling has shown that the structure of glycogen is optimized for its ability to store and deliver energy quickly and for the longest amount of time possible. The key to this optimization is the average chain length of the branches (13 residues). If the average chain length were much greater or much shorter, glycogen would not be as efficient a vehicle for energy storage and release on demand. Experimental results support the conclusions reached from the mathematical modeling.
How does the breakdown of glycogen take place?
Glycogen is found primarily in liver and muscle. The release of
glycogen stored in the liver is triggered by low levels of glucose in blood.
Liver glycogen breaks down to glucose-6-phosphate, which is hydrolyzed to give
glucose. The release of glucose from the liver by this breakdown of glycogen
replenishes the supply of glucose in the blood. In muscle, glucose-6-phosphate
obtained from glycogen breakdown enters the glycolytic pathway directly rather
than being hydrolyzed to glucose and then exported to the bloodstream.
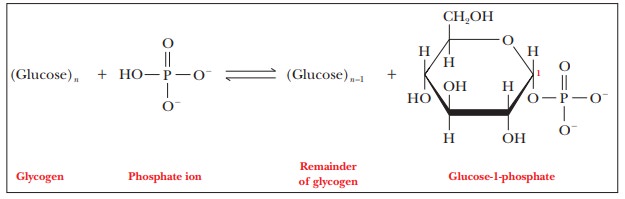
Three reactions play roles in the conversion of glycogen to
glucose-6-phosphate. In the first reaction, each glucose residue cleaved from
glycogen reacts with phosphate to give glucose-1-phosphate. Note particularly
that this cleavage reaction is one of phosphorolysis
rather than hydrolysis.
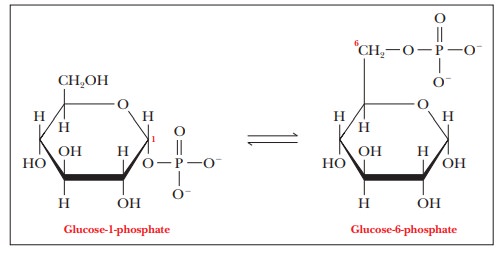
In a second reaction, glucose-1-phosphate isomerizes to give glucose-6-phosphate.
Complete breakdown of glycogen also requires a debranching reaction
to hydrolyze the glycosidic bonds of the glucose residues at branch points in
the glycogen structure. The enzyme that catalyzes the first of these reactions
is glycogen phosphorylase; the
second reaction is catalyzed by phosphoglucomutase.
Glycogen phosphorylase
Glycogen + Pi OO3 =>
Glucose-1-phosphate + Remainder of glycogen
Phosphoglucomutase
Glucose-1-phosphate =>
Glucose-6-phosphate

Glycogen phosphorylase cleaves the α(1 - > 4) linkages in glycogen. Complete
breakdown requires debranching enzymes
that degrade the α(1 -
> 6) linkages. Note that no ATP is hydrolyzed in the first reaction. In the
glycolytic pathway, we saw another example of phosphorylation of a substrate
directly by phosphate without involvement of ATP: the phosphorylation of
glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate.
This is an alternative mode of entry to the glycolytic pathway that “saves” one
molecule of ATP for each molecule of glucose because it bypasses the first step
in glycolysis. When glycogen rather than glucose is the starting material for
glycolysis, there is a net gain of three ATP molecules for each glucose
monomer, rather than two ATP molecules, as when glucose itself is the starting
point. Thus, glycogen is a more effective energy source than glucose. Of
course, there is no “free lunch” in biochemistry and, as we shall see, it takes
energy to put the glucoses together into glycogen.
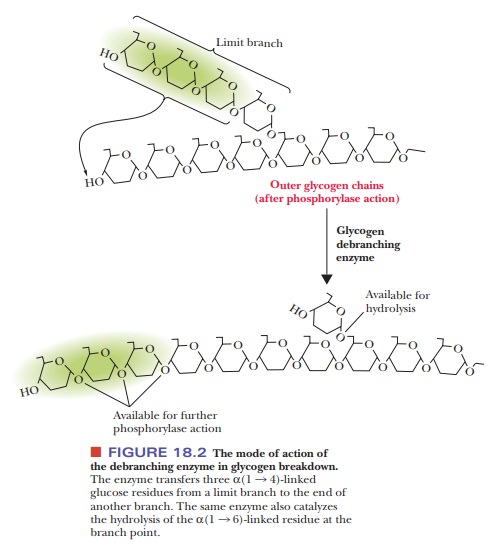
The debranching of glycogen involves the transfer of a “limit
branch” of three glucose residues to the end of another branch, where they are
subse-quently removed by glycogen phosphorylase. The same glycogen debranching
enzyme then hydrolyzes the α(1 - > 6) glycosidic bond of the last
glucose resi-due remaining at the branch point (Figure 18.2).
When an organism needs energy quickly, glycogen breakdown is
important. Muscle tissue can mobilize glycogen more easily than fat and can do
so anaero-bically. With low-intensity exercise, such as jogging or
long-distance running, fat is the preferred fuel, but as the intensity
increases, muscle and liver glyco-gen becomes more important. Some athletes,
particularly middle-distance run-ners and cyclists, try to build up their
glycogen reserves before a race by eating large amounts of carbohydrates.
How is glycogen formed from glucose?
The formation of glycogen from glucose is not the exact reversal of
the breakdown of glycogen to glucose. The synthesis of glycogen requires
energy, which is provided by the hydrolysis of a nucleoside triphosphate, UTP.
In the first stage of glycogen synthesis, glucose-1-phosphate (obtained from
glucose-6-phosphate by an isomerization reaction) reacts with UTP to produce
uridine diphosphate glucose (also called UDP-glucose or UDPG) and pyrophosphate
(PPi).
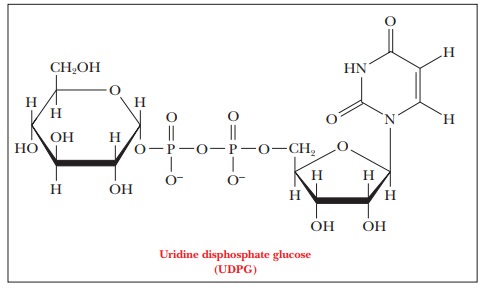
The enzyme that catalyzes this reaction is UDP-glucose pyrophosphorylase. The exchange of one phosphoric
anhydride bond for another has a free-energy change close to zero. The release
of energy comes about when the enzyme inorganic pyrophosphatase catalyzes the
hydrolysis of pyrophosphate to two phosphates, a strongly exergonic reaction.
It is common in biochemistry to see the energy released by the
hydrolysis of pyrophosphate combined with the free energy of hydrolysis of a
nucleoside triphosphate. The coupling of these two exergonic reactions to a
reaction that is not energetically favorable allows an otherwise endergonic
reaction to take place. The supply of UTP is replenished by an exchange
reaction with ATP, which is catalyzed by nucleoside phosphate kinase:
UDP + ATP < - - - > UTP + ADP
This exchange reaction makes the hydrolysis of any nucleoside
triphosphate energetically equivalent to the hydrolysis of ATP.
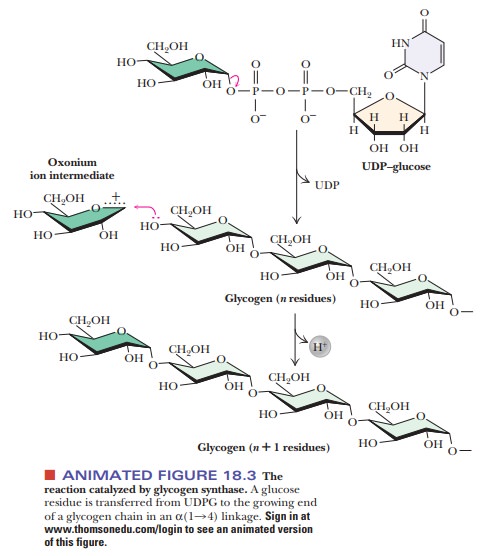
The addition of UDPG to a growing chain of glycogen is the next
step in glycogen synthesis. Each step involves formation of a new α(1 - > 4) glycosidic bond
in a reaction catalyzed by the enzyme glycogen
synthase (Figure 18.3). This enzyme cannot simply form a bond between two
isolated glucose mol-ecules; it must add to an existing chain with α(1 - > 4) glycosidic
linkages. The initiation of glycogen synthesis requires a primer for this
reason. The hydroxyl group of a specific tyrosine of the protein glycogenin (37,300 Da) serves this
purpose. In the first stage of glycogen synthesis, a glucose residue is linked
to this tyrosine hydroxyl, and glucose residues are successively added to this
first one. The glycogenin molecule itself acts as the catalyst for addition of
glucoses until there are about eight of them linked together. At that point,
glycogen synthase takes over.

Synthesis of glycogen requires the formation of α(1 - > 6) as well as α(1 - > 4) glycosidic
linkages. A branching enzyme
accomplishes this task. It does so by transferring a segment about 7 residues
long from the end of a growing chain to a branch point where it catalyzes the
formation of the required α(1 - > 6) gly
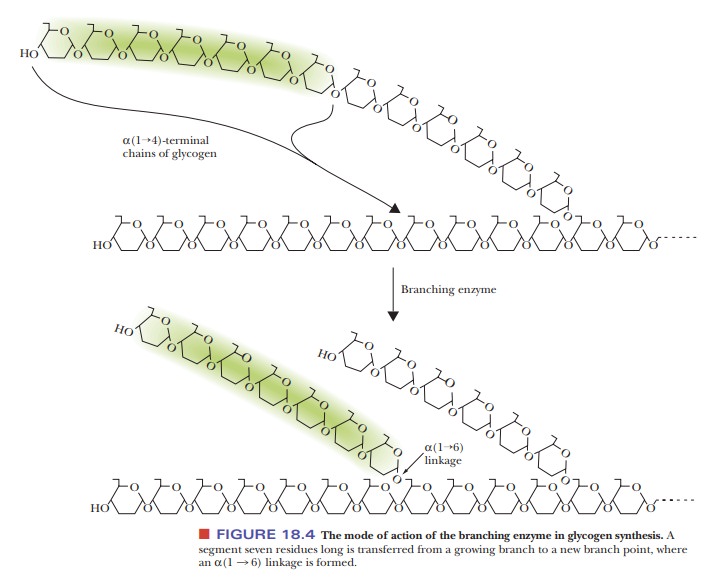
Note that this enzyme has already
catalyzed the breaking of an α(1 - > 4) glycosidic linkage in the process
of transferring the oligosaccharide segment. Each transferred segment must come
from a chain at least 11 residues long; each new branch point must be at least
4 residues away from the nearest existing branch point.
How is glycogen metabolism controlled?
How does an organism ensure that glycogen synthesis and glycogen
breakdown do not operate simultaneously? If this were to occur, the main result
would be the hydrolysis of UTP, which would waste chemical energy stored in the
phosphoric anhydride bonds. A major controlling factor lies in the behavior of
glycogen phosphorylase. This enzyme is subject not only to allosteric control but
also to another control feature: covalent modification. In that example,
phosphorylation and dephosphorylation of an enzyme determined whether it was
active, and a similar effect takes place here.
Figure 18.5 summarizes some of the salient control features that affect glyco-gen phosphorylase activity. The enzyme is a dimer that exists in two forms, the inactive T (taut) form and the active R (relaxed) form. In the T form (and only in the T form), it can be modified by phosphorylation of a specific serine resi-due on each of the two subunits. The esterification of the serines to phosphoric acid is catalyzed by the enzyme phosphorylase kinase; the dephosphorylation is catalyzed by phosphoprotein phosphatase. The phosphorylated form of glycogen phosphorylase is called phosphorylasea, and the dephosphorylated form is called phosphorylaseb. The switch from phosphorylase b to phosphorylase a is the major form of control over the activity of phosphorylase. The response time of the changes is on the order of seconds to minutes. Phosphorylase is also controlled more quickly in times of urgency by allosteric effectors, with a response time of milliseconds.
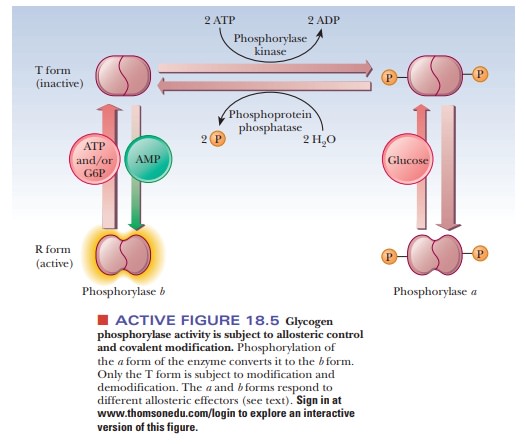
In liver, glucose is an allosteric inhibitor of
phosphorylase a. It binds to the
substrate site and favors the transition to the T state. It also exposes the
phosphorylated serines so that the phosphatase can hydrolyze them. This shifts
the equilibrium to phosphorylase b.
In muscle, the primary allosteric effectors are ATP, AMP, and
glucose-6-phosphate (G6P). When the muscles use ATP to contract, AMP levels
rise. This increase in AMP stimulates formation of the R state of phosphorylase
b, which is active. When ATP is
plentiful or glucose-6-phosphate builds up, these molecules act as allosteric
inhibitors shifting the equilibrium back to the T form. These differences
ensure that glycogen will be degraded when there is a need for energy, as is
the case with high [AMP], low [G6P], and low [ATP]. When the reverse is true
(low [AMP], high [G6P], and high [ATP]), the need for energy, and consequently
for glycogen breakdown, is less. “Shutting down” glycogen phosphorylase
activity is the appropriate response. The combination of covalent modification
and allosteric control of the process allows for a degree of fine-tuning that
would not be possible with either mechanism alone. Hormonal control also enters
into the picture. When epinephrine is released from the adrenal gland in
response to stress, it triggers a series of events, that suppress the activity
of glycogen synthase and stimulate that of glycogen phosphorylase.
The activity of glycogen synthase is subject to
the same type of covalent modification as glycogen phosphorylase. The
difference is that the response is opposite. The inactive form of glycogen
synthase is the phosphorylated form. The active form is unphosphorylated. The
hormonal signals (glucagon or epi-nephrine) stimulate the phosphorylation of
glycogen synthase via an enzyme called cAMP-dependent protein kinase. After the
glycogen syn-thase is phosphorylated, it becomes inactive at the same time the
hormonal sig-nal is activating phosphorylase. Glycogen synthase can also be
phosphorylated by several other enzymes, including phosphorylase kinase and
several enzymes called glycogen synthase kinases. Glycogen synthase is
dephosphorylated by the same phosphoprotein phosphatase that removes the
phosphate from phos-phorylase. The phosphorylation of glycogen synthase is also
more complicated in that there are multiple phosphorylation sites. As many as
nine different amino acid residues have been found to be phosphorylated. As the
progressive level of phosphorylation increases, the activity of the enzyme
decreases.
Glycogen synthase is also under allosteric
control. It is inhibited by ATP. This inhibition can be overcome by
glucose-6-phosphate, which is an activa-tor. However, the two forms of glycogen
synthase respond very differently to glucose-6-phosphate. The phosphorylated
(inactive) form is called glycogensynthase
D (for “glucose-6-phosphate dependent”) because it is active onlyunder very
high concentrations of glucose-6-phosphate. In fact, the level necessary to
give significant activity would be beyond the physiological range. The
nonphosphorylated form is called glycogen
synthase I (for “glucose-6-phosphate independent”) because it is active
even with low concentrations of glucose-6-phosphate. Thus, even though purified
enzymes can be shown to respond to allosteric effectors, the true control over
the activity of glycogen syn-thase is by its phosphorylation state, which, in
turn, is controlled by hormonal states.
The fact that two target enzymes, glycogen
phosphorylase and glycogen syn-thase, are modified in the same way by the same
enzymes links the opposing processes of synthesis and breakdown of glycogen
even more intimately.
Finally, the modifying enzymes are themselves subject to covalent modifica-tion and allosteric control. This feature complicates the process considerably but adds the possibility of an amplified response to small changes in conditions. A small change in the concentration of an allosteric effector of a modifying enzyme can cause a large change in the concentration of an active, modified target enzyme; this amplification response is due to the fact that the substrate for the modifying enzyme is itself an enzyme.
At this point, the situation has become very complex indeed, but it is a good
example of how opposing pro-cesses of breakdown and synthesis can be controlled
to the advantage of an organism.
Summary
Glycogen is the storage form of glucose in animals, including
humans. Glycogen releases glucose when energy demands are high.
Glucose polymerizes to form glycogen when the organism has no
immedi-ate need for the energy derived from glucose breakdown.
Glycogen metabolism is subject to several
different control mechanisms, including covalent modification and allosteric
effects.
Related Topics