Chapter: Biochemistry: Storage Mechanisms and Control in Carbohydrate Metabolism
Gluconeogenesis Produces Glucose from Pyruvate
Gluconeogenesis Produces Glucose
from Pyruvate
The conversion of pyruvate to glucose occurs by a process called gluconeogenesis. Gluconeogenesis is not
the exact reversal of glycolysis. We first met pyruvate as a product of
glycolysis, but it can arise from other sources to be the starting point of the
anabolism of glucose. Some of the reactions of glycolysis are essentially irreversible;
these reactions are bypassed in gluconeogenesis. An analogy is a hiker who goes
directly down a steep slope but climbs back up the hill by an alternative,
easier route. We shall see that the biosynthesis and the degradation of many
important biomolecules follow different pathways.
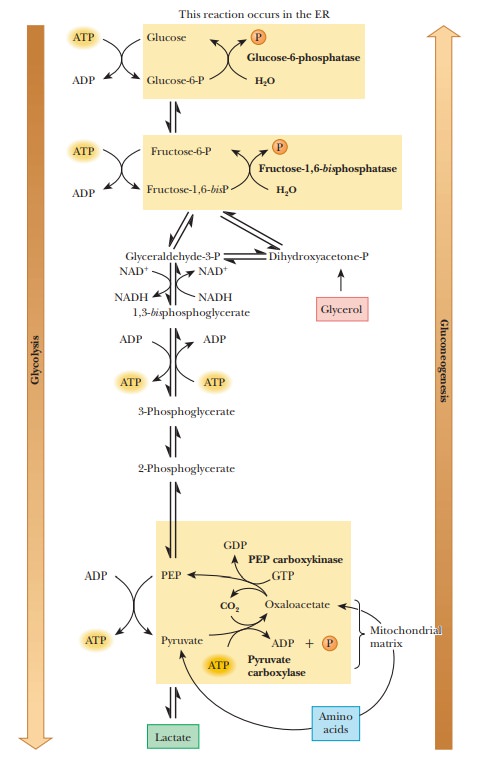
Glycolysis involves three irreversible steps, and the differences
between glycolysis and gluconeogenesis are found in these three reactions. The
first of the glycolytic reactions is the production of pyruvate (and ATP) from
phosphoenolpyruvate. The second is the production of fructose-1,6-bisphosphate from fructose-6-phosphate,
and the third is the production of glucose-6-phosphate from glucose. Because
the first of these reactions is exergonic, the reverse reaction is endergonic.
Reversing the second and third reactions would require the production of ATP
from ADP, which is also an endergonic reaction. The net result of
gluconeogenesis includes the reversal of these three glycolytic reactions, but
the pathway is different, with different reactions and different enzymes
(Figure 18.6).
Why is oxaloacetate an intermediate in gluconeogenesis?
The conversion of pyruvate to phosphoenolpyruvate in
gluconeogenesis takes place in two steps. The first step is the reaction of
pyruvate and carbon dioxide to give oxaloacetate. This step requires energy,
which is available from the hydrolysis of ATP.
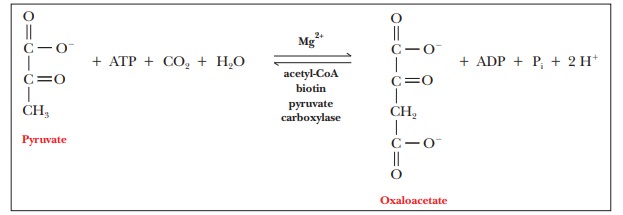
The
enzyme that catalyzes this reaction is pyruvate
carboxylase, an allosteric enzyme found in the mitochondria. Acetyl-CoA is
an allosteric effector that activates pyruvate carboxylase. If high levels of
acetyl-CoA are present (in other words, if there is more acetyl-CoA than is
needed to supply the citric acid cycle), pyruvate (a precursor of acetyl-CoA)
can be diverted to gluconeogenesis. (Oxaloacetate from the citric acid cycle
can frequently be a starting point for gluconeogenesis as well.) Magnesium ion
(Mg2+) and biotin are also required for effective catalysis. We have
seen Mg2+ as a cofactor before, but we have not seen biotin, which
requires some discussion.
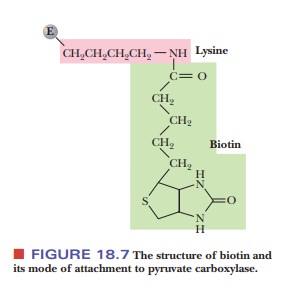
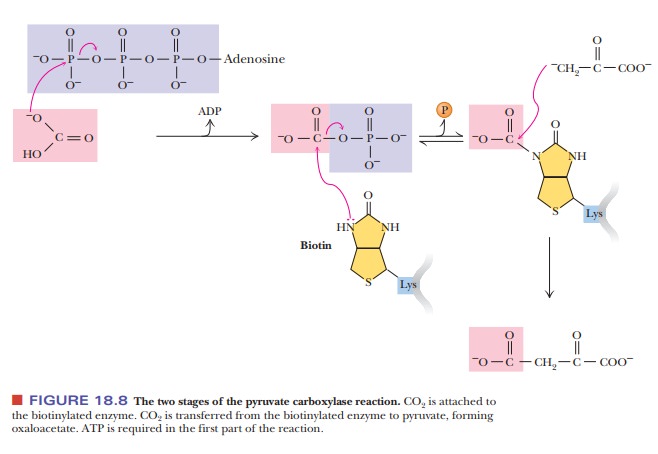
Biotin is a carrier of carbon dioxide; it has a
specific site for covalent attach-ment of CO2 (Figure 18.7). The
carboxyl group of the biotin forms an amide bond with the ε-amino group of a specific lysine side chain of pyruvate
carbox-ylase. The CO2 is attached to the biotin, which, in turn, is
covalently bonded to the enzyme, and then the CO2 is shifted to
pyruvate to form oxaloacetate (Figure 18.8). Note that ATP is required for this
reaction.
The conversion of oxaloacetate to phosphoenolpyruvate is catalyzed by the enzyme phosphoenolpyruvate carboxykinase (PEPCK), which is found in the mitochondria and the cytosol.
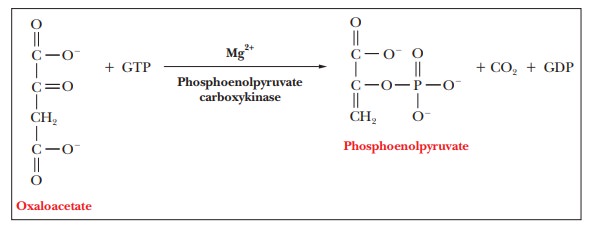
This reaction also involves hydrolysis of a nucleo-side triphosphate—GTP, in this case, rather than ATP.
The
successive carboxylation and decarboxylation reactions are both close to
equilibrium (they have low values of their standard free energies); as a
result, the conversion of pyruvate to phosphoenolpyruvate is also close to
equilibrium ( ∆G°' = 2.1 kJ mol–1
= 0.5 kcal mol–1). A small increase in the level of oxaloacetate can
drive the equilibrium to the right, and a small increase in the level of
phosphoenolpyruvate can drive it to the left. A concept well known in general
chemistry, the law of mass action,
relates the concentrations of reactants and products in a system at
equilibrium. Changing the concentration of reactants or products causes a shift
to reestablish equilibrium. A reaction proceeds to the right on addition of
reactants and to the left on addition of products.
Pyruvate
+ ATP + GTP - > Phosphoenolpyruvate + ADP + GDP + Pi
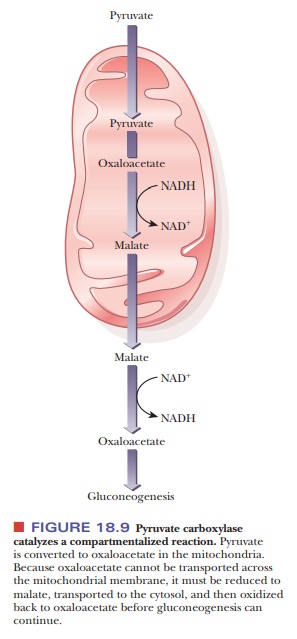
The oxaloacetate formed in the mitochondria can have two fates with
respect to gluconeogenesis. It can continue to form PEP, which can then leave
the mitochondria via a specific transporter to continue gluconeogenesis in the
cytosol. The other possibility is that the oxaloacetate can be turned into
malate via mitochondrial malate dehydrogenase, a reaction that uses NADH, as
shown in Figure 18.9. Malate can then leave the mitochondria and have the reaction
reversed by cytosolic malate dehydrogenase. The reason for this two-step
process is that oxaloacetate cannot leave the mitochondria, but malate can.
(The pathway involving malate is the one that takes place in the liver, where
gluconeogenesis largely takes place.) You might wonder why these two paths
exist to get PEP into the cytosol to continue gluconeogenesis. The answer
brings us back to a familiar enzyme we saw in glycolysis,
glyceraldehyde-3-phosphate dehydrogenase. Remember that the purpose of lactate
dehydrogenase is to reduce pyruvate to lactate so that NADH could be oxidized
to form NAD+, which is needed to continue glycolysis. This reaction
must be reversed in gluconeogenesis, and the cytosol has a low ratio of NADH to
NAD+. The purpose of the roundabout way of getting oxaloacetate out
of the mitochondria via malate dehydrogenase is to produce NADH in the cytosol
so that gluconeogenesis can continue.
What is the role of sugar phosphates in gluconeogenesis?
The other two reactions in which gluconeogenesis differs from
glycolysis are ones in which a phosphate-ester bond to a sugar-hydroxyl group
is hydrolyzed. Both reactions are catalyzed by phosphatases, and both reactions
are exergonic. The first reaction is the hydrolysis of fructose-1,6-bisphosphate to produce
fructose-6-phosphate and phosphate ion ( ∆G°'
= –16.7 kJ mol–1 = –4.0 kcal mol–1).
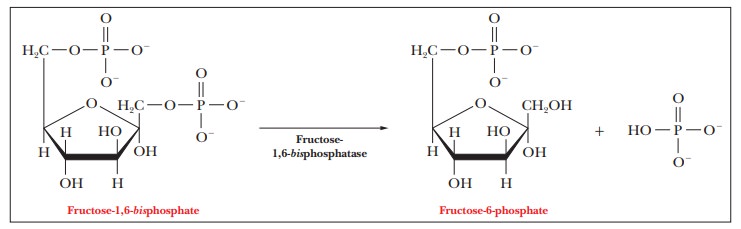
This reaction is catalyzed by the enzyme fructose-1,6-bisphosphatase,
an allosteric enzyme strongly inhibited by adenosine monophosphate (AMP) but
stimulated by ATP. Because of allosteric regulation, this reaction is also a
control point in the pathway. When the cell has an ample supply of ATP, the
formation rather than the breakdown of glucose is favored.
The second reaction is the hydrolysis of glucose-6-phosphate to
glucose and phosphate ion ( ∆G°' =
–13.8 kJ mol–1 = –3.3 kcal mol–1). The enzyme that cata-
lyzes this reaction is glucose-6-phosphatase.
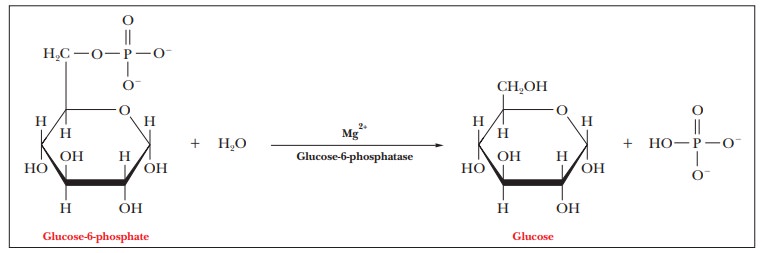
When we discussed glycolysis, we saw that both of the
phosphorylation reactions, which are the reverse of these two
phosphatase-catalyzed reactions, are endergonic. In glycolysis, the phosphorylation
reactions must be coupled to the hydrolysis of ATP to make them exergonic and
thus energetically allowed. In gluconeogenesis, the organism can make direct
use of the fact that the hydrolysis reactions of the sugar phosphates are
exergonic. The corresponding reactions are not the reverse of each other in the
two pathways. They differ from each other in whether they require ATP and in
the enzymes involved. Hydrolysis of glucose-6-phosphate to glucose occurs in
the endoplasmic reticulum. This is an example of an interesting pathway that
requires three cellular locations (mitochondria, cytosol, endoplasmic
reticulum).
Summary
Glucose is formed from pyruvate, which in turn can be obtained from
lac-tate that accumulates in muscle during exercise. This process, called
glu-coneogenesis, takes place in the liver after lactate is transported there
by the blood. The newly formed glucose is transported back to the muscles by
the blood.
Gluconeogenesis
by passes the irreversible reactions
of glycolysis. Oxaloacetate is an intermediate in this
pathway.
Related Topics