Chapter: 11th Biochemistry : Chapter 3 : Proteins
Haemoglobin - an example for globular protein
Haemoglobin - an example for globular protein
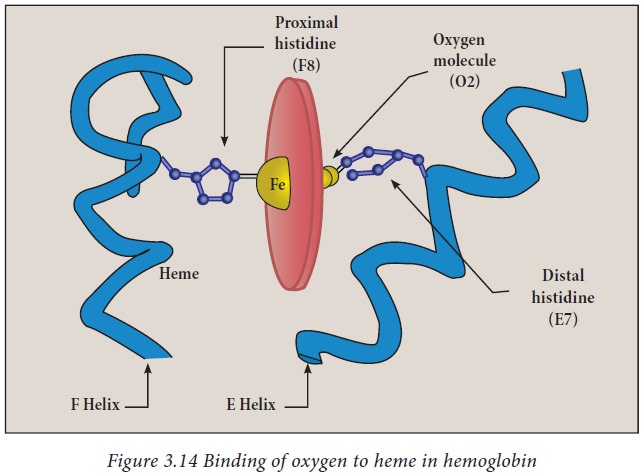
Haemoglobin
is found in red blood cells and is involved in the transport of oxygen from
lungs to tissues. It is a tetramer containing four polypeptide chains – 2α
chains and 2 β chains. Each of these chains contains a prosthetic group called
heme. Heme is a protoporphyrin ring complexed with Fe2+. This Fe 2+
ion can form six bonds, four with the nitrogen atoms of the porphyrin ring, one
with a histidine of hemoglobin and the other with oxygen. Thus every
haemoglobin can carry four O2 molecules.
Haemoglobin
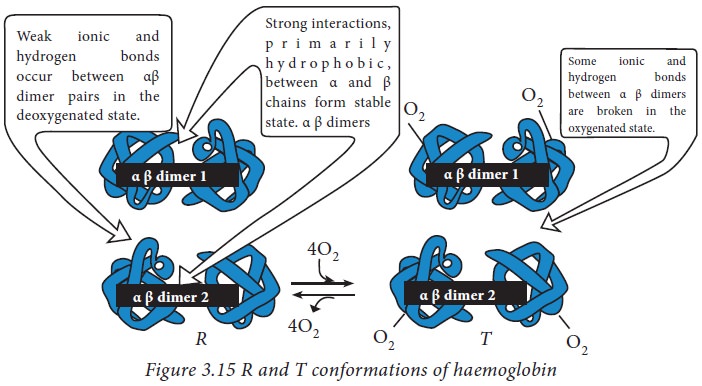
is an alpha helical protein, meaning it does not contain β sheets as its
secondary structural elements. The haemoglobin tetramer structure could be
considered as a dimerized dimer of (αβ)1 and (αβ)2. The α and β chain in each
dimer are held together strongly by hydrophobic interactions. The interactions
between (αβ)1 and (αβ)2 are comparatively weaker hydrogen bonds and ionic
interactions. This allows the dimers to move with respect to each other forming
two different conformational states: a relaxed ‘R’ conformation and a taut ‘T’
conformation. The binding and release of oxygen switches the hemoglobin between
these two states.
Haemoglobinopathies are a set of
diseases caused by synthesis of structurally abnormal haemoglobins, insufficient
amount of haemoglobins or both. Sickle cell anemia, thalasemia, porphyria etc
are examples of haemoglobinopathies.
Related Topics