Chapter: 11th Biochemistry : Chapter 3 : Proteins
Collagen - an example for fibrous protein
Collagen - an example for fibrous protein
Collagen is the most abundant protein
found in humans. Unlike globular structures discussed above, collagen forms a
long coiled fibrous structure. Each collagen molecule consists of three
polypeptide chains which forms an elongated triple helical structure as
described in figure 3.16
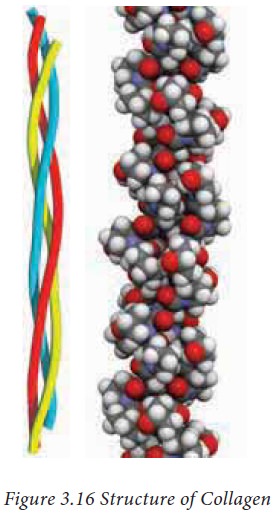
The amino acid sequence of these
polypeptide chains are always repeating units of Gly-X-Y. Where X is often
proline and Y is either hydroxyproline or hydroxylysine. The hydroxyl group of
hydroxylysine can also be glycosylated with glucose or galactose.
There are various types of collagen
which can be broadly categorized into three groups.
a. The fibril forming collagens
present in skin, bone, cartilages, tendons and blood vessels etc provide
tensile strength to the corresponding tissues.
b. The network forming collagens form
network like structures beneath the membranes providing them mechanical
strength.
c. The fibril associated collagens
connect two fibril forming collagens or a fibril forming collagen with other
components of extra cellular matrix.
More than 1000 disease causing
mutations have been identified both directly in collagen genes or in the genes
of enzymes involved in the synthesis of collagen. The diseases associated with
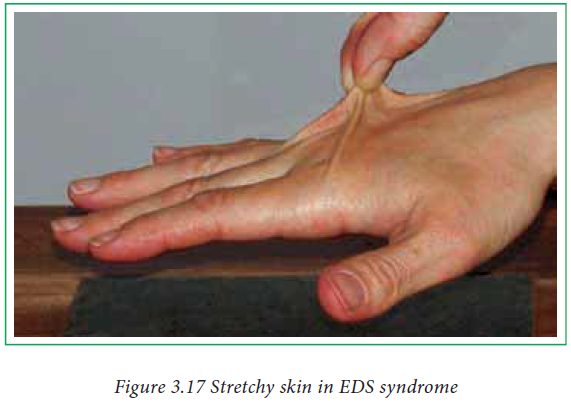
collagen malfunction are known as collagenopathies. Elhers-Danlos syndrome
(EDS) is a prominent collagenopathy which is due to inherited mutations in
collagen processing enzymes. Stretchy skin, loose joints and vascular problems
are associated with EDS.
Osteogenesis Imperfecta (O.I) is
another prominent collagenopathy characterized by brittle bones, hunch back,
twisted spine and retarded wound healing. The mutations in glycine residues of
collagen and thereby an improper triple helical structure leads to this
disease.
Related Topics