Chapter: Essentials of Psychiatry: Anxiety Disorders: Generalized Anxiety Disorder
Generalized Anxiety Disorder: Pharmacotherapy
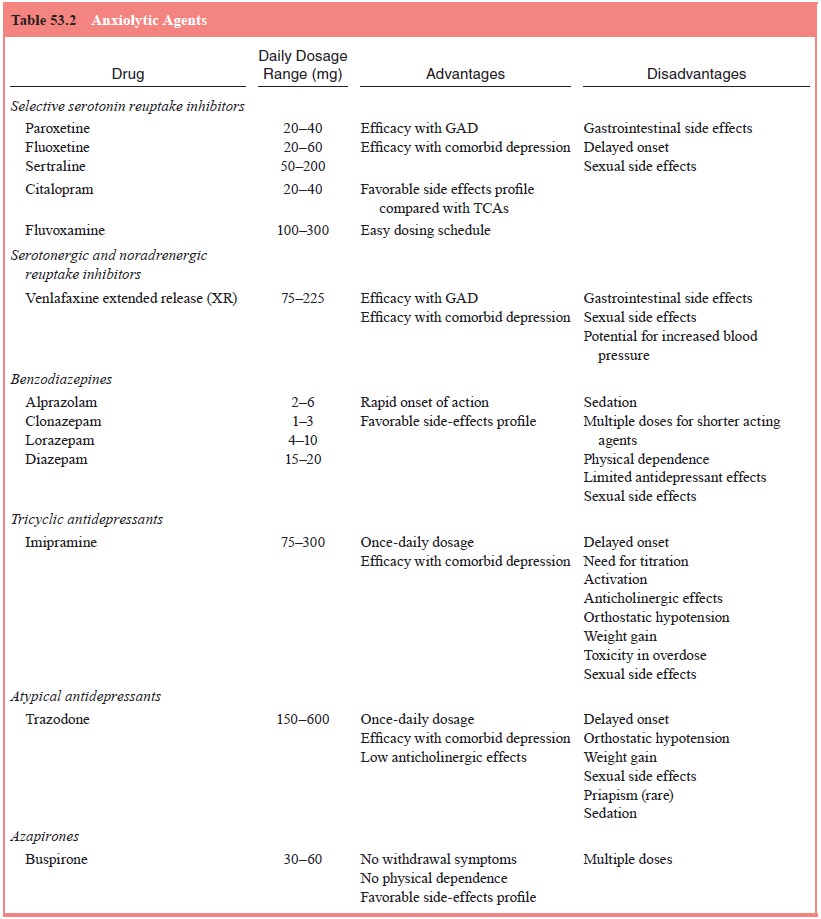
Pharmacotherapy
Below, we will discuss the use of various anxiolytic agents in the
treatment of GAD. Table 53.2 provides a summarization of this information.
Benzodiazepines
Benzodiazepines are commonly used for the treatment of GAD and are still
considered by some clinicians to be the first-line treatment for GAD. Several
controlled studies have demonstrated the efficacy of different benzodiazepines
such as diazepam, chlordiazepoxide and alprazolam in the treatment of GAD. The
available placebo-controlled studies found that diazepam, alpra-zolam and
lorazepam were effective in the treatment of GAD. The benzodiazepines have a
broad spectrum of effects includ-ing sedation, muscle relaxation, anxiety
reduction and decreased physiologic arousal (e.g. palpitations, tremulousness,
etc.). Inter-estingly, available studies indicate that benzodiazepines have the
most pronounced effect on hypervigilance and somatic symp-toms of GAD, but exhibited
fewer effects on psychic symptoms such as dysphoria, interpersonal sensitivity
and obsessionality (Hoehn-Saric et al.,
1988).
The main difference between individual benzodiazepines is potency and
elimination half-life. These differences may have important treatment
implications. For example, benzo-diazepines with relatively short elimination
half-lives such as alprazolam (range of 10–14 hours) may require dosing at
least three to four times a day in order to avoid interdose symptom rebound.
Conversely, the use of longer-acting compounds such as clonazepam (range of
20–50 hours) may minimize the risk of interdose symptom recurrence. In
comparative studies of differ-ent benzodiazepines, alprazolam appeared to
perform somewhat better than lorazepam. Data from the HARP study indicate that
the most frequently reported medication used by GAD patients was alprazolam
(31%), followed by clonazepam (23%) (Yonkers et al., 1996).
Benzodiazepines exert their therapeutic effects quickly, often after a
single dose. However, concern has emerged over the use of benzodiazepines,
particularly long-term benzodiazepine use. Side effects of benzodiazepines,
such as sedation, psycho-motor impairment and memory disruption, were noted by
treating clinicians, and confirmed in research studies. Further, although it
was suggested that the use pattern of benzodiazepines by pa-tients with anxiety
disorders may not represent abuse, addiction, or drug dependence as typically
understood, the chronic use of benzodiazepines in the treatment of GAD has been
increasingly discouraged in recent years.
When initiating treatment with benzodiazepines, it is helpful for patients
to take an initial dose at home in the evening to see how it affects them.
Gradual titration to an effective dose allows for limiting unwanted adverse
effects. A final daily dos-age of alprazolam between 2 and 4 mg/day, 1 and 2
mg/day for clonazepam, or 15 and 20 mg/day of diazepam is usually suf-ficient
for the majority of patients. Upon treatment discontinu-ation, it is important
to consider appropriate taper in order to avoid withdrawal symptoms. Possible
factors that may contrib-ute to the severity of withdrawal and the ultimate
outcome of benzodiazepine taper include the dosage, duration of treatment, the
benzodiazepine elimination half-life and potency, and the rate of
benzodiazepine taper (gradual versus abrupt). Addition-ally, patient factors such
as premorbid personality features have been implicated. It appears that a taper
rate of 25% per week is probably too rapid for many patients. We, therefore,
recommend a slow benzodiazepine taper of at least 4 to 8 weeks, with the final
50% of the taper conducted even more gradually, with the patient decreasing by
the lowest possible daily dose of the ben-zodiazepines during this period. We
also recommend continu-ing to use divided doses of short to intermediate
half-life ben-zodiazepines (alprazolam, lorazepam) during taper to minimize
fluctuations in benzodiazepine levels over a 24-hour period, or using longer
half-life benzodiazepines (such as clonazepam) which have the advantage of
maintaining a once- or twice-daily dosing schedule.
Tricyclic Antidepressants
Clinical trials conducted in the early 1990s have confirmed that tricyclic antidepressants (TCAs) may also be effective in the treatment of GAD. These studies, as well as other trials, suggest that TCAs may be especially effective in the treatment of psychic symptoms of GAD (Brawman-Mintzer and Lydiard, 1994).The relationship between plasma levels of TCAs and their anxiolytic efficacy in patients with GAD has not been studied. Until this relationship is clarified, decisions regarding the total daily doses and the monitoring of plasma levels should be based on the patient’s treatment response and side-effects profile. Fur-ther, due to potential jitteriness, restlessness and agitation during the initial stages of treatment, we suggest that the ini-tial dose of the TCAs in patients with GAD may need to be low (for example 10 mg/day of imipramine), and increased gradually.
Adverse effects commonly associated with the use of TCAs include
anticholinergic effects (dry mouth, blurred vision, constipation),
cardiovascular effects (orthostatic hypotension, slightly increased heart
rate), sexual side effects and weight gain. As mentioned, patients may also
experience significant jitteriness, restlessness and agitation during the
initial stages of treatment. These side-effects often limit the acceptability
of TCAs by many patients. Potential toxicity in overdose has been of concern to
clinicians as well. Due, in part, to the side-effect profile, need for dose
titration and importantly the emergence of new and effective agents (as
described below), the use of TCAs in the treatment of GAD has been reserved for
those resistant to these newer agents.
Serotonin Reuptake Inhibitors
Selective serotonin reuptake inhibitors (SSRIs) are rapidly be-coming a
key tool in the treatment of GAD. SSRIs are generally well tolerated. The most
problematic side effect associated with SSRI use is interference with sexual
function (e.g., delayed or-gasm or abnormal ejaculation) in women and men. A
variety of treatment strategies have been suggested for the management of
SSRI-induced sexual dysfunction. Such strategies include wait-ing for tolerance
to develop, dosage reduction, drug holidays and various augmentation strategies
with 5-hydroxytryptamine-2 (5-HT2), 5-HT3 and alpha-2-adrenergic receptor antagonists, 5-HT1A and dopamine receptor agonists,
and phosphodiesterase (PDE5) enzyme inhibitors.
Serotonergic and Noradrenergic Reuptake Inhibitors
The antidepressant venlafaxine extended release (XR) is an in-hibitor of
both 5-HT and NE reuptake (SNRI). Several large, placebo-controlled trials have
evaluated it in the treatment of patients with DSM-IV-diagnosed GAD. As a
result, venlafaxine XR was the first antidepressant approved by the FDA for the
treat-ment of GAD. Results from two short-term studies indicate that
venlafaxine XR (75, 150 and 225 mg/day) was significantly more effective than
placebo and superior to buspirone on certain anxi-ety measures and in the
prevention of relapse (Sheehan, 2001). The adverse events for GAD patients
treated with venlafaxine XR resembled those in depression trials. The most
common ad-verse events included nausea, somnolence, dry mouth, dizziness,
sweating, constipation and anorexia.
Other Antidepressants
Both trazodone and imipramine have comparable efficacy to di-azepam. In
addition, trazodone and imipramine exhibit higher efficacy in the treatment of
psychic symptoms such as tension, apprehension and worry. The
alpha-2-adrenoreceptor antagonist mitrazapine, which is also a 5-HT2, 5-HT3 and H(1) receptors an-tagonist,
has been evaluated as a potential anxiolytic in the treat-ment of patients with
major depressive disorder and comorbid GAD in an 8-week, open-label study
(Goodnick et al., 1999). Re-sults
suggest that this antidepressant may be useful in the treat-ment of anxiety
symptoms.
Azapirones
Buspirone hydrochloride, the only currently marketed aza-pirone, was the
first nonbenzodiazepine anxiolytic agent ap-proved for the treatment of
persistent anxiety by FDA. Re-sults have been mixed about the efficacy of
buspirone over placebo. For example, in four placebo-controlled studies that
compared buspirone with a standard benzodiazepine, two showed no benefit for
diazepam and buspirone over placebo, and two showed no benefit for buspirone
over placebo. Benzo-diazepines may also be slightly more effective than
buspirone in the treatment of somatic symptoms of anxiety but no signif-icant
differences appear to exist between buspirone and ben-zodiazepines in measures
of psychic anxiety (Rickels et al.,
1997). Side effects most frequently associated with buspirone use included
gastrointestinal system-related side effects, such as appetite disturbances and
abdominal complaints, and diz-ziness. Prior use of benzodiazepines may
adversely affect the therapeutic response to buspirone. DeMartinis and
colleagues (2000) found that buspirone treatment was less effective for
patients who had been taking benzodiazepines within 30 days of initiating
buspirone treatment. Delle Chiaie and colleagues (1995) reported that a gradual
2-week taper of lorazepam with a simultaneous addition of buspirone for 6 weeks
prevents the development of clinically significant rebound anxiety or
benzodiazepine withdrawal. This approach was shown to pro-vide clinically
significant relief of anxiety symptoms in GAD patients previously treated with
benzodiazepines for 8 to 14 weeks.
Perhaps the most significant problem with the use of buspirone has been
that experts have advocated too low a dose to produce symptom reduction. In
order to achieve optimal re-sponse, buspirone dosing in the range of at least
30 to 60 mg/day is currently recommended.
Other Agents
Hydroxyzine is a histamine-1 receptor blocker and a muscarinic receptor
blocker. Recent controlled trials with the antihistamine have suggested that
this compound may be effective in the acute treatment of GAD symptoms. Finally,
the potential use of the an-ticonvulsant gabapentin in the treatment of anxiety
symptoms has also been suggested.
Related Topics