Chapter: Essential Anesthesia From Science to Practice : Clinical management : General anesthesia
General anesthesia
General anesthesia
General anesthesia requires many preparatory steps. These include the pre-operative evaluation of the patient and the procurement and preparation of all equipment to be used, drugs to be given, intravenous cannulae to be inserted for the infusion of the necessary fluids, monitors, and the tools and techniques needed for the establishment of an open airway. Elsewhere in this you will find all of these topics addressed. Here, we will limit ourselves to a discussion of how to induce and maintain general anesthesia and how to ease the patient out of the drug-induced coma before transfer to the post-anesthesia care unit (PACU).
Induction, maintenance and emergence
Once the preparations for general anesthesia are complete, the patient’s history and physical examination are reviewed, the machine and equipment are set up and tested, the patient is on the table, and the monitors are applied, we are ready to send the patient on one of the strangest journeys of his life: general anesthesia. We will administer drugs by injection and inhalation that will take possession of the patient’s body. If we have used neuromuscular blocking agents, ventilation will cease, and the patient will be unable to move. In short, such an unconscious patient will have been reduced to a physiologic organism without a will.
To appreciate the enormity of this statement, consider the extreme of this con-dition: once general anesthesia has been established for some cardiac proce-dures, we might lower the patient’s temperature to the point where all currently monitored variables cease to show evidence of life. There will be no heartbeat, no electrocardiogram, no spontaneous breathing, and the electroencephalogram will show no deflection. There will be no reflex, no motion, and no reaction to any intervention. If, at this point, you were to bring in an observer, unaware of what had been done, he might well pronounce the patient dead. And yet, if we raise the temperature and initiate mechanical ventilation, the patient’s cardiac and respiratory function will slowly resume their own life and, once the temperature approaches normal and the effects of drugs wear off, the patient will wake up. You might ask searching questions about the patient’s state, his personality, his soul during this approach to death. We cannot imagine a more profound responsibility than that of the anesthesiologist taking a patient to such an extreme approxima-tion to death while guarding his life.
In routine general anesthesia we do not drive the system to the just described extreme. Yet, a defenseless patient under general anesthesia will expect the anes-thesiologist to stand in for him and his dignity and attend to him with focused attention and great skill.
During general anesthesia, we must provide the patient with sleep, amnesia, and analgesia; we must monitor his vital signs and keep them within physiologic limits, and we must make the surgeon’s task as easy as possible with the double benefit of helping the surgeon so that she can do her best for the patient. But before we start general anesthesia, an intravenous infusion (usually Ringer’s lactate) is running, and we often give intravenously an anxiolytic with amnesic power such as midazolam1 (1 to 2 mg for the average adult) and/or a narcotic, such as fentanyl (50 to 100 mcg for the average adult). Some like to give the narcotic even though the patient has no pain and even though the drug will not cause euphoria. Instead, it can serve as a gentle background and preemptive analgesic for the operation and, by weakening (but not eliminating) the sympathetic response, it can smooth out swings of blood pressure and heart rate during intubation. We always keep in mind the synergistic respiratory depression of a mixture of benzodiazepines and opioids.
Pre-oxygenation
The establishment of a patent airway is probably our most important safety concern. Disaster overtakes the patient within a matter of minutes if he cannot breathe for himself (because we paralyzed him), and we cannot ventilate his lungs (because his airway is obstructed by soft tissue and because we cannot intubate his trachea for any number of reasons). Then minutes, even seconds, count. If, before inducing apnea, we replace the nitrogen in his lungs with oxygen, we can gain 3 to 6 minutes (more with a large functional residual capacity (FRC)) before arterial hypoxemia occurs. Therefore, we routinely pre-oxygenate patients before inducing anesthesia. This procedure is simple: we apply a face mask and select a flow of oxygen high enough to prevent the patient from inhaling his exhaled nitrogen. The latter is vented and, after about 3 minutes, the patient’s FRC will contain very little nitrogen, much oxygen, and the usual amount of water vapor and carbon dioxide.
Induction
We now introduce hypnotic, analgesic, and anesthetic drugs into the body either by intravenous injection or via the lungs (in the past intramuscularly or even rectally). While inhalation anesthesia can be induced without the help of intra-venous drugs, the most common approach is to inject a fast-acting drug such as thiopental (3 to 5 mg/kg) or propofol (1 to 3 mg/kg). Within a couple of minutes, these drugs will reach their peak effect, at which time intubation of the trachea becomes feasible, usually with the help of muscle relaxants such as succinyl-choline. Neither thiopental nor propofol offers relaxation of muscles or analge-sia. Therefore, they are wonderful for gentle induction but would be unlikely to provide adequate operating conditions for an intra-abdominal procedure.
Instead of intubating the trachea, we have the option of inserting a laryngeal mask airway (LMA), which does not require the use of a muscle relaxant and is par-ticularly welcome when the patient need not be intubated at all and is breathing spontaneously throughout the operation (see Airway management chapter).
Once we have placed the endotracheal tube or LMA and have confirmed its proper location by auscultation and end-tidal CO2, we can begin the adminis-tration of inhalation, intravenous (TIVA, total intravenous anesthesia) or a com-bination anesthetic. A number of halogenated drugs are available (halothane, isoflurane, desflurane, sevoflurane), but we use only one at a time. Each can be given together with 50–70% nitrous oxide in oxygen. Nitrous oxide provides modest analgesic background without cardiovascular depression to speak of. Surgical anesthesia (the patient will not respond to the incision) can be obtained within a matter of minutes so that the induction of anesthesia need not delay the incision.
Propofol is the poster child agent for TIVA. Purported advantages of this tech-nique are shortened wake-up and PACU times, and reduced risk of postoperative nausea and vomiting. Rather than halogenated agents, patients for outpatient surgery might receive a propofol infusion (for sedation and sleep) with nitrous oxide to provide a modicum of analgesia and ensure amnesia, supplemented with small amounts of analgesics.
The rapid sequence induction
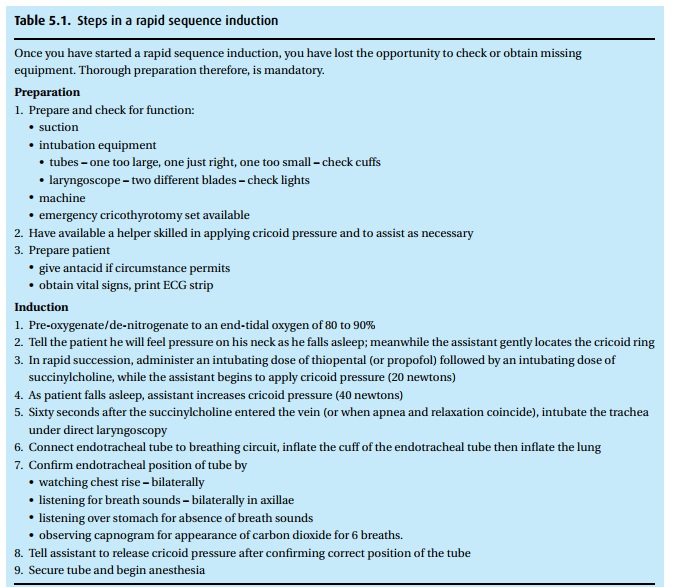
Patients who need general anesthesia, even though they have a full stomach (hav-ing recently eaten or having a condition that interferes with gastric emptying such as trauma or pregnancy), require a special technique, the so-called rapid sequence induction (Table 5.1). With a full stomach, the specter of regurgitation and aspir-ation arises. The technique calls for a thorough denitrogenation, followed by the administration of thiopental and succinylcholine in rapid succession while we maintain pressure on the cricoid ring (the so-called Sellick maneuver2). Remem-ber, the cricoid is the only ring of the trachea that does not have a membrane posteriorly and, instead, is cartilaginous throughout its circumference. So, push-ing on it compresses the esophagus. You can feel the cricoid ring just under the larynx. Only once we have confirmed the proper position of the endotracheal tube and inflated the cuff can we stop the Sellick maneuver.
Positioning
For many operations, the patient can lie on his back. Others require positions that may take an hour or more to be accomplished (for example, neurosurgical operations). We need to understand what position favors access for the surgeon and what positions present dangers for the patient (interference with ventila-tion, compression of nerves, extreme flexion or extension of joints). Thus, the positioning is often a joint surgical/anesthesia task during which a lot of foam padding finds application between patient and hard surfaces. The most common post-operative nerve palsy affects the ulnar nerve (funny bone), which is exposed to pressure, being superficial and running through the ulnar groove at the elbow (between the medial epicondyle and the olecranon).
Depth of anesthesia and monitoring
Once the patient is positioned, we must keep the anesthetic level so that the patient will neither feel pain nor remember the operation. Yet this “anesthetic depth” must be balanced against the hemodynamic consequences (hypotension) of excess anesthetic, as well as the potential for delayed wake-up. If the patient is not paralyzed, there will be little doubt that he will move and let us know if he feels pain. We need to gauge the depth of anesthesia clinically and with the help of instruments. The clinical assessment includes monitoring heart rate and blood pressure, which should be neither high from sympathetic response to noxious stimulation, nor low from overdose with anesthetics. In recent years processed EEG signals have become available that claim to help gauge the depth of anesthesia by generating a score linked to EEG activity, which becomes depressed as anesthesia deepens. In addition to these signals we keep track of the intravenous drugs the patient has had, of their effects and duration, and of the concentration of expired anesthetics, which reflect blood and finally brain levels. Thus the conduct of general anesthesia calls for continual attention to a number of parameters and variables.
At the same time, we monitor pulse oximetry, blood pressure, heart rate, ECG, tidal volume, respiratory rate and peak inspiratory pressure, inspired oxygen, the concentration of respired gases and vapors, and the capnogram. Should blood loss, deep anesthesia, surgical activity (for example compressing the vena cava), an embolism (for example, air aspirated in an open vein), or a process originating in the patient (such as anaphylaxis or coronary insufficiency) cause a problem, we should be able to discover the effects as early as possible so that we can take corrective actions. We also assess the degree of muscle relaxation with the help of a nerve stimulator (twitch monitor) and by watching the operation and gauging muscle tone, which might impede the surgeon’s work. Thus, we cannot be satisfied with watching the monitors; we need to keep an eye on the patient, his face, his position, and the surgeon’s work.
A tedious aspect of our work is the obligation to keep a record of all these events and of our activities, such as the administration of drugs and fluids, adjustment of ventilator settings, and even of surgical events (“aorta clamped at 9:24 am!”). Automated record keeping systems are becoming increasingly sophisticated.
Emergence
Well before the surgeon puts in the last stitch, we begin preparation for having the patient wake up. This might call for the reversal of a non-depolarizing neuromuscular blocking drug and the scaling back of inspired anesthetic con-centrations. Furthermore, our goal is to have the patient awaken quickly and without pain; therefore, we titrate opioids or our regional anesthetic to anticipate the pain level without unacceptable respiratory depression, while also consider-ing the risk for postoperative nausea and vomiting. It is a fine art to gauge the surgical process and the patient’s requirements so that the patient opens his eyes when the dressing goes on. “Hello,” we say, and, after confirming the patient is strong, able to protect his airway (gag reflex), breathing and following commands, we suction his airway and say, “All done! Let me take out that tube,” when we pull the endotracheal tube or the LMA. While the patient is not likely to remember such words, they provide a fitting ending to a perfect anesthetic!
We then accompany the patient to the Post-Anesthesia Care Unit (PACU) where we go through a formal process of turning the care of the patient over to a spe-cialized PACU nurse, unless the patient is fit for early discharge home or needs to be admitted to the Intensive Care Unit.
Problems
Things don’t always run smoothly. If critical incidents occur, they must be dis-covered and corrected in time, lest they lead to disasters. To catch early trends, however, presents more difficulties than one might think, because most signals we monitor are rather non-specific. Thus, a low SpO2 could be the result of malignant hyperthermia or faulty hospital piping, or low blood pressure the consequence of bleeding, deep anesthesia, or a measuring artifact. Therefore, with any deviation from normal, we need to think holistically about the patient and the anesthesia system with all of its components.
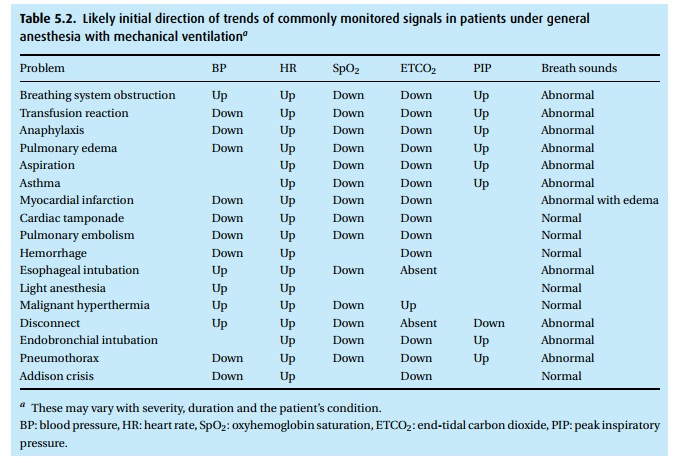
In Table 5.2, we have listed trends in various monitored parameters as they often appear during certain problems. Observe two points:
· Usually we cannot arrive at a diagnosis by simply looking at the monitors. We need additional information, which we must urgently collect when trends herald trouble.
· Breath and heart sounds turn out to be very helpful. Always listen to heart and lungs (wheezing, crackling, uneven breath sounds – or, importantly – normal breath sounds) to include or exclude certain items from a differential diagnosis. Equally important is the peak inspiratory pressure.
To complicate matters even further, not all patients react in an identical manner. Co-existing diseases can obscure changes or reverse direction of an expected change. Finally, trends can reverse direction, depending on how long the problem existed and how grave the incident. For example, hypercarbia and hypoxemia secondary to inadequate ventilation because of obstruction in the endotracheal tube can first cause sympathetic stimulation and a rise in blood pressure and heart rate. However, if the problem persists, pressure will decline and, with severe hypoxemia, extreme bradycardia can supervene.
Malignant hyperthermia: Patients with this rare (≈1:20 000) inherited defect in intracellular calcium control are asymptomatic until given succinylcholine or anesthetic vapors, which can trigger a violent increase in metabolism with sky-rocketing O2 consumption and CO2 production. Tachycardia and rapidly rising ETCO2 precede by many minutes a murderous fever. High creatine kinase levels reflect extensive muscle damage. Immediate cooling and i.v. dantrolene have greatly improved the prognosis. Triggering agents must be avoided subsequently.
Related Topics