Chapter: Mechanical : Engineering Thermodynamics : Gas Mixtures and Psychrometry
Gas Mixtures
GAS MIXTURES
Pure substance
is defined as a substance which means that it is homogeneous and unchanging in
chemical composition.
COMPOSITION OF A
GAS MIXTURE: MASS AND MOLE FRACTIONS
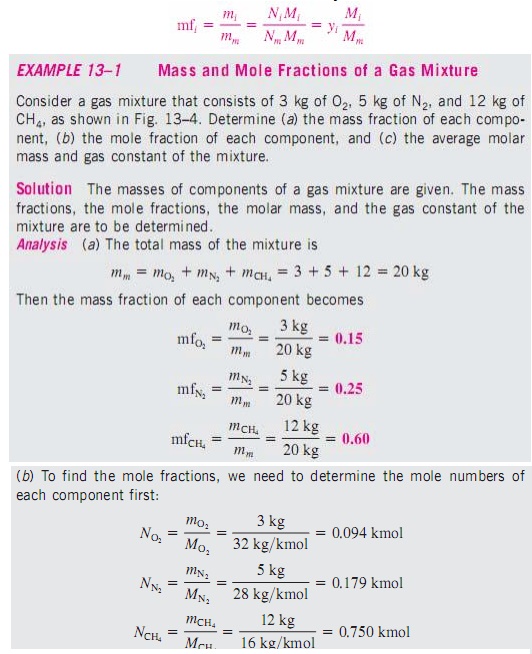
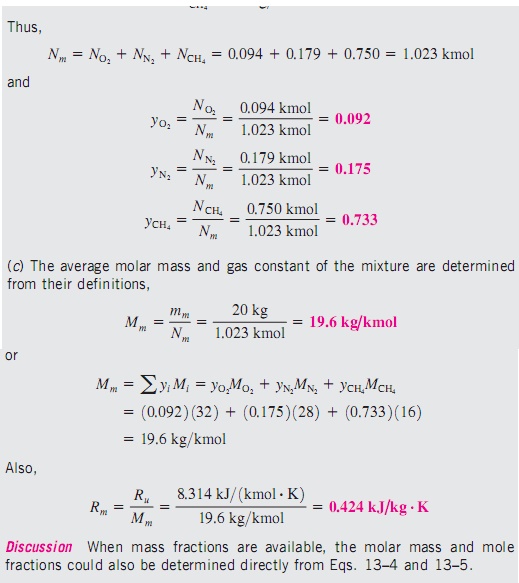
To determine the properties
of a mixture, we need to know the composition of the mixture as well as the
properties of the individual components. There are two ways to describe the
composition of a mixture: either by specifying the number of moles of each
component, called molar analysis, or by specifying the mass of each component,
called gravimetric analysis.
Consider
a gas mixture composed of k components. The mass of the mixture mm is the sum
of the masses of the individual components, and the mole number of the mixture
Nm is the sum of the mole numbers of the individual components.
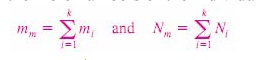
The
ratio of the mass of a component to the mass of the mixture is called the mass
fraction mf, and the ratio of the mole number of a component to the mole number
of the mixture is called the mole fraction
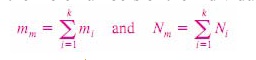
Dividing
(1) by mm or (2) by Nm, we can easily show that the sum of the mass fractions
or mole fractions for a mixture is equal to 1
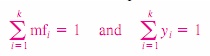
The
mass of a substance can be expressed in terms of the mole number N and molar
mass M of the substance as m _ NM. Then the apparent (oraverage) molar mass and
the gas constant of a mixture can be expressed as
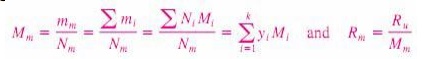
The
molar mass of a mixture can also be expressed as
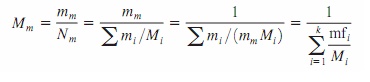
Mass
and mole fractions of a mixture are related by
P-v-T BEHAVIOR
OF GAS MIXTURES: IDEAL AND REAL GASES
An ideal gas is defined
as a gas whose molecules are spaced far apart so that the behavior of a
molecule is not influenced by the presence of other molecules—a situation
encountered at low densities. We also mentioned that real gases approximate
this behavior closely when they are at a low pressure or high temperature
relative to their critical-point values. The P-v-T behavior of an ideal gas is
expressed by the simple relation Pv _ RT, which is called the ideal-gas
equation of state. The P-v-T behavior of real gases is expressed by more
complex equations of state or by Pv _ ZRT, where Z is the compressibility
factor.
When
two or more ideal gases are mixed, the behavior of a molecule normally is not
influenced by the presence of other similar or dissimilar molecules, and
therefore a nonreacting mixture of ideal gases also behaves as an ideal gas.
Air, for example, is conveniently treated as an ideal gas in the range where
nitrogen and oxygen behave as ideal gases. When a gas mixture consists of real
(nonideal) gases, however, the prediction of the P-v-T behavior of the mixture
becomes rather involved.
The
prediction of the P-v-T behavior of gas mixtures is usually based on two
models: Dalton’s law
of additive pressures
Both models are described and discussed
below.
Dalton’s law of
additive pressures:
Dalton's
law (also
called Dalton's law of partial pressures) states that the total pressure
exerted by a gaseous mixture is equal to the sum of the partial pressures of
each individual component in a gas mixture. This empirical law was observed by
John Dalton in 1801 and is related to the ideal gas laws. Mathematically, the
pressure of a mixture of gases can be defined as the summation
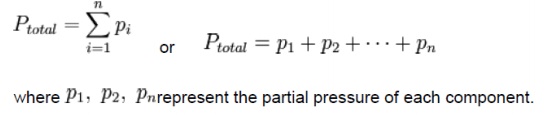
Amagat’s law of
additive volumes:
Amagat's
law or
the Law of Partial Volumes of 1880 describes the behavior and properties
of mixtures of ideal (as well as some cases of non-ideal) gases. Of use in
chemistry and thermodynamics, Amagat's law states that the volume Vm of
a gas mixture is equal to the sum of volumes Vi of the K
component gases, if the temperature T and the pressure p remain
the same:
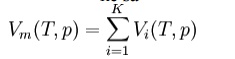
This
is the experimental expression of volume as an extensive quantity. It is named
after Emile Amagat. Both Amagat's and Dalton's Law predict the properties of
gas mixtures. Their predictions are the same for ideal gases. However, for real
(non-ideal) gases, the results differ. Dalton's Law of Partial Pressures
assumes that the gases in the mixture are non-interacting (with each other) and
each gas independently applies its own pressure, the sum of which is the
total pressure. Amagat's Law assumes that the volumes of each component
gas (same temperature and pressure) are additive; the interactions of the
different gases are the same as the average interactions of the components.
Heat capacity
The
heat capacity at constant volume of an ideal gas is:
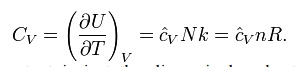
It is seen that the
constant is just the dimensionless heat capacity at constant volume. It is
equal to half the number of degrees of freedom per particle. For
moderate temperatures, the constant for
a monoatomic gas is Cv = 3/2 while for
a
diatomic gas it Is Cv = 5/2 . It is seen
that macroscopic measurements on heat capacity provide information on the
microscopic structure of the molecules.
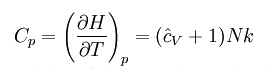
where
H = U + pV is the enthalpy of the gas. It is seen that is
also a constant and that the dimensionless heat capacities are related by:
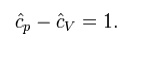
ENTROPY
Using
the results of thermodynamics only, we can go a long way in determining the
expression for the entropy of an ideal gas. This is an important step since,
according to the theory of thermodynamic potentials, of which the internal
energy U is one, if we can express the entropy as a function of U
and the volume V, then we will have a complete statement of the
thermodynamic behavior of the ideal gas. We will be able to derive both the
ideal gas law and the expression for internal energy from it. Since the entropy
is an exact differential, using the chain rule, the change in entropy when
going from a reference state 0 to some other state with entropy S may be
written asS where:
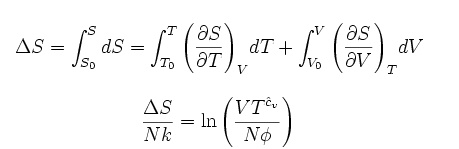
This is about as far as
we can go using thermodynamics alone. Note that the above equation is flawed —
as the temperature approaches zero, the entropy approaches negative infinity,
in contradiction to the third law of thermodynamics. In the above
"ideal" development, there is a critical point, not at absolute zero,
at which the argument of the logarithm becomes unity, and the entropy becomes
zero.
Related Topics