Chapter: Mechanical : Engineering Thermodynamics : Ideal And Real Gases, Thermodynamic Relations
Important Questions and Answers: Ideal And Real Gases, Thermodynamic Relations
IDEAL AND REAL GASES, THERMODYNAMIC
RELATIONS
1. State
Avogadro’s law?
Avogadro’s law states, “Equal volumes of
d and pressure, contain equal number of molecu
2. State
Dalton’s law partial pressure?
Dalton’s law of partial pressure state,”
T sum of the partial pressures exerted by individual gases if each one of them
occupied
separately
in the total volume of the mixture at mixture temperature”.
3. How
does the Vander waals equation differ from the ideal gas equation of state?
·
Intermolecular attractive study is made.
·
Shape factor is considered.
These assumptions are not made in ideal gas equation
of state.
4. What
is meant by virtual expansion?[A
Viral or Virtual expansions are only
applicable to gases of low and medium densities. The equation state of a
substance is given by,
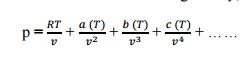
The coefficient
ofevirala(T),coefficients. Theb(T),c(T)viralcoefficientwill…. ar vanish when
the pressure becomes zero. Finally, the equation of state reduces to the
ideal-gas
equation.
5. Distinguish between ideal and real gas?
An ideal gas is one which strictly
follows the gas laws under all conditions of temperature and pressure.
In actual practice, there is no real gas
which strictly follows the gas laws over the entire range of temperature and
pressure. However, hydrogen, oxygen, nitrogen and air behave as an ideal gas
under certain temperature and pressure limits.
6. What
are Maxwell relations?
There
are four Maxwell relations,
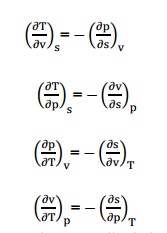
These
are the Maxwell relations.
7. Define Joule–Thomson co–efficient?
Joule –Thomson co –efficient is defined
as the change in temperature with change in pressure, keeping the enthalpy
remains constant. It is denoted by,
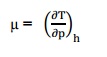
7. Define
co-efficient of volume expansion and isothermal compressibility?
Co-efficient of volume expansions is defined as the
change in volume with change in
temperature
per unit volume keeping the pressure constant. It is denoted by,
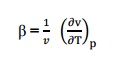
Isothermal
compressibility is defined as the change in volume with change in pressure per
unit volume by keeping the temperature constant. It is denoted by,
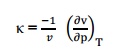
8. What
is compressibility factor?
We know that, the perfect gas equation is pv=RT. But
for real gas, a correction factor has
![]()
![]()
to be introduced in the perfect gas equation to take
into account the deviation of real gas from the perfect gas equation. This
factor is known as compressibility factor (Z) and is defined by,
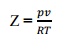
10.
What is compressibility factor?
What does it signify? What is its value for an ideal das at critical point?
We know that, the perfect gas equation
is pv=RT. But for real gas, a correction factor has to be introduced in the
perfect gas equation to take into account the deviation of real gas from the
perfect gas equation. This factor is known as compressibility factor
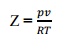
(Z)
and is defined by,
·
Intermolecular attractive study is made.
·
Shape factor is considered.
At critical
point,ation the Vander
waal’s equ

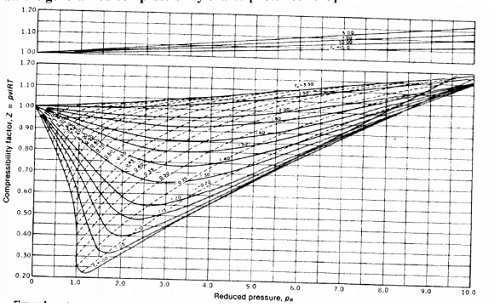
10.
Draw the generalized
compressibility chart?
![]()
12.
What is Joule Thomson coefficient?
Why is it zero for an ideal gas?
Joule –Thomson co –efficient is defined as the
change in temperature with change in pressure, keeping the enthalpy remains
constant. It is denoted by,
We know that the equation of state as pV
=RT
Differentiating the above equation of state with
respect to T by keeping pressure, p constant.
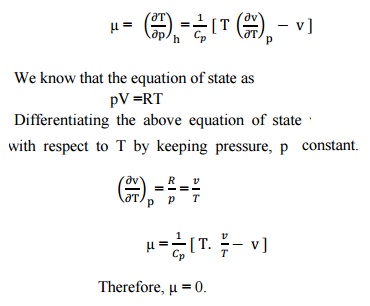
Therefore,
µ = 0.
It
implies that the Joule-Thomson coefficient is zero for ideal gas.
13.
What is Clasius Clapeyron Equation?
Clapeyron equation involves relationship
between the saturation temperature, saturation pressure, the enthalpy of
evaporation and the specific volume of the two phases involved.
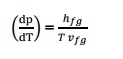
14.
State Tds Equations?
Tds Equations are,
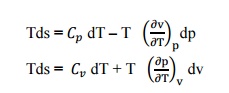
15.
State Helmholtz function?
Helmholtz function is property of a
system and is given by subtracting the product of absolute temperature (T) and
entropy (s) from the internal energy u.
i.e.
Helmholtz function = u –Ts
16.
Explain the Amagat’slaw of partial volume.
The total volume of mixture of gases is
equal to the sum of the partial volumes of the components. The partial volume
of a component will be the volume it will occupy at the total pressure at that
temperature.
17.
Explain Boyle’s law.
It states that for given mass of gas at
constant temperature, the pressure is inversely proportional to its volume.
18.
Explain Charle’s law.
It states that for a given mass of gas
at constant pressure, its volume varies directly as its absolute temperature.
19.
Explain Gay Lussac’s
law.
It states that for a given mass of gas
at constant volume, its pressure is directly proportional to its absolute
temperature
Related Topics