Chapter: Mechanical : Engineering Thermodynamics : Gas Mixtures and Psychrometry
Solved Problems: Gas Mixtures and Psychrometry
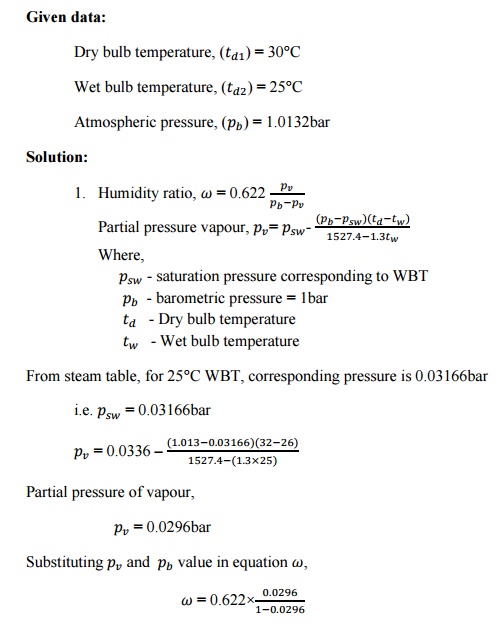
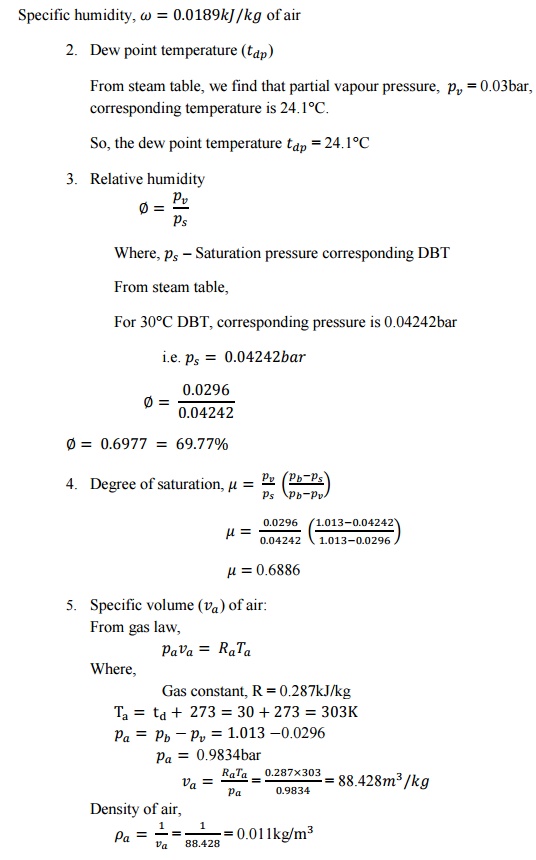
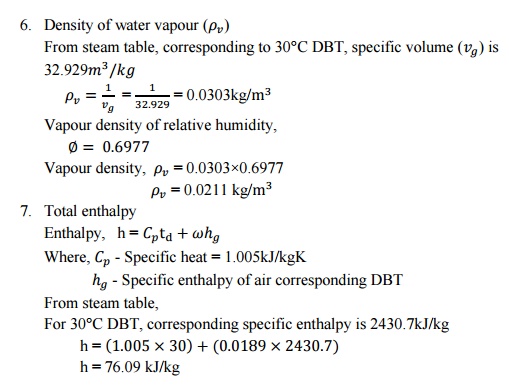
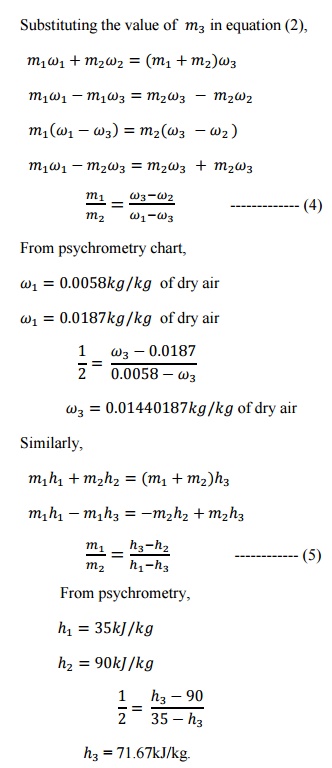
SOLVED PROBLEMS
1. Atmospheric
air at 1.0132bar has a DBT of 30°C and a WBT of 25°C. Compute
i.
The partial pressure of water
vapour,
ii.
The specific humidity,
iii.
The dew point temperature,
iv.
The relative humidity,
v.
The degree of saturation,
vi.
The density of air in the mixture,
vii.
The density of vapour in the
mixture and
viii.
The enthalpy of the mixture. Use
thermodynamic table.
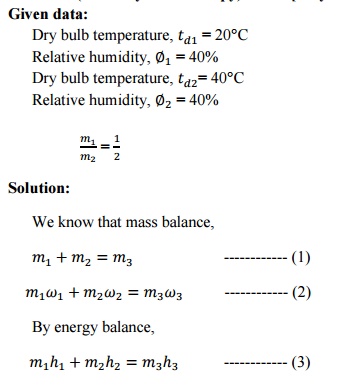
2. Air at 20°C, 40% relative
humidity is missed adiabatically with air at 40°C, 40% RH in the ratio of 1kg of
former with 2kg of latter (on dry basis). Find the final condition (humidity
and enthalpy) of air.
3.An air conditioning system is to
take in outdoor air at 283K and 30percent
relative humidity at a steady rate of 45 /min and to condition it to
298K and 60% relative humidity. The outdoor air is first heated to 295K in the
heating section and then humidified by the injection of hot steam in the
humidifying section. Assuming the entire process takes place at a pressure of
100kPa, determine (i) the rate of heat supply in the heating section and (ii)
the mass flow rate of the steam required in the humidifying section.
Given data:
= 10°C
= 30%
= 25°C
= 60%
= 22°C
= 45 /min = 100kPa
Solution:
Step 1:
The dry of air i.e 10°C
of dry bulb temperature and 30% relative humidity is marked on the
psychrometric chart at point
1. The
horizontal line is drawn up to 22°C to obtain point
2. The
dry of air i.e. 25°C dry bulb temperature and 60% relative humidity is marked
on the psychrometric chart at point
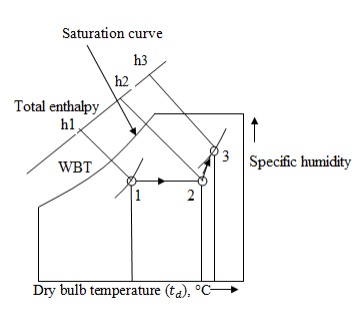
Draw
an inclined line from point 1 to 2. Read enthalpies and specific humidity
values
at point 1,2 and 3 from psychrometric chart.
At
point 1, enthalpy =
16.5kJ/kg
At
point 2, enthalpy =
28kJ/kg
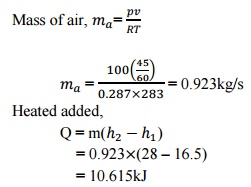
Mass
of air,=
=
0.923kg/s
Heated
added,
= 0.923 (28 –16.5)
= 10.615kJ
Specific
humidity,
= 0.003kg/kg
of dry air
= 0.012kg/kg
of dry air
Moisture
added,
=
0.012 –0.003 = 0.009kg/kg of dry air
We
know that,
Specific
humidity,
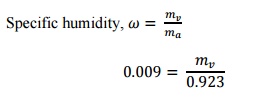
Mass
of steam =
0.0083kg/s
4. (i)
What is the lowest temperature that air can attain in an evaporative cooler, if
it enters at 1atm, 302K and 40% relative humidity? [Nov/Dec 2008]
Given data: p
= 1bar T = 302K = 40%
Solution:
From steam table,
corresponding to dry bulb temperature 29°C, saturation pressure is 4.004kPa
Relative
humidity,
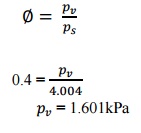
=
1.601kPa
Lowest temperature that
air attain in an evaporative cooler = 113.6°C which is corresponding to =
1.601kPa.
(ii)
Consider a room that contains air at 1atm, 308K and 40% relative humidity.
Using the psychrometric chart, determine: the specific humidity, the enthalpy,
the wet bulb temperature, the dew point temperature and the specific volume of
air.
Given data:
Pressure,
p = 1atm
Relative humidity, = 40% Temperature, T
= 308K = 35°C
Solution:
· Specific humidity,
From the point 1, draw a horizontal line
with respect to temperature and relative humidity. At this point specific
humidity is 0.0138kJ/kg. i.e. = 0.0138kJ/kg
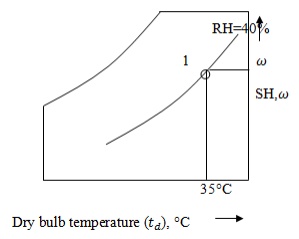
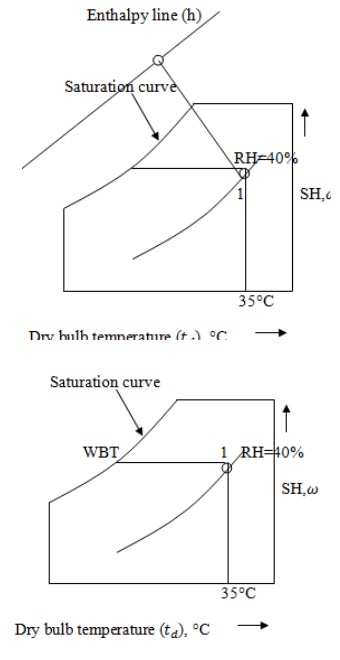
· Enthalpy, h and
Wet bulb temperature,
From the point 1, draw
a inclined line along the constant wet bulb temperature line till it cuts
enthalpy line. At this point, enthalpy is 52.5kJ/kg and wet bulb temperature is
23.9°C. i.e. h = 52.5kJ/kg and
=
23.9°C
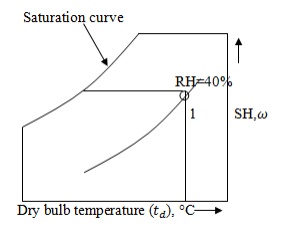
Mark point 1 on the
psychrometric chart by given dry bulb temperature (35°C) and relative humidity
40%.
From point 1, draw a horizontal line to
the left till it cuts saturation curve.
At that point, temperature
is 20°C and specific volume is 0.89 .
i.e.
= 20°C and v = 0.89 .
5. 30
/min of moist air at 15°C DBT and 13°C WBT are mixed with 12 /min of moist air
at 25°C DBT and 18°C WBT. Determine DBT and WBT of the mixture assuming
barometric pressure is one atmosphere.
Given data:
First steam of air,
Dry bulb temperature, = 15°C
Wet bulb temperature, = 13°C
Flow rate, = 30 /min
Second steam of air,
Dry bulb temperature, = 25°C
Wet bulb temperature, = 18°C
Flow rate, = 12 /min
Solution:
Step
1:
The first steam of air
15°C DBT and 13°C WBT is marked on the psychrometric chart at point 1.
Step
2:
The second stream of
air 25°C DBT and 18°C WBT is marked on the psychrometric chart at point 2.
Join the points 1 and 2 from
psychrometric chart. Step 3:
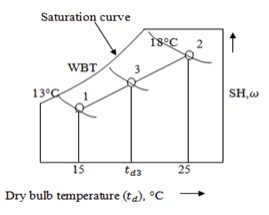
We
know that,
Specific humidity of
first steam of air, = 0.007kg/kg of dry air
Specific humidity of second steam of
air,

= 0.00943kg/kg of dry
air Specific humidity after mixing,
=
0.00943kg/kg of dry air
Step
5:
Draw a horizontal line from = 0.00943 till it cuts 1 –2 line.
Name
the point 3.
From
psychrometric chart, at point 3
Dry bulb temperature, = 24.02°C
Wet bulb temperature, = 18.2°C
Related Topics