Chapter: Obstetrics and Gynecology: Cancer of the Uterine Corpus
Evaluation - Endometrial Hyperplasia
EVALUATION
Histologic evaluation of a sample
of the endometrium establishes the diagnosis of endometrial hyperplasia or
carcinoma. Endometrial biopsy is
most easily accom-plished by any number of different atraumatic aspiration
devices used in the office.
The
diagnostic accuracy of office endometrial biopsy is 90% to 98%, compared with
dilation and curettage (D&C) or hysterectomy.
The routine Pap smear is not
reliable in diagnosing endometrial hyperplasia or cancer, as only 30% to 40% of
patients with endometrial carcinoma have abnormal Pap test results. On the
other hand, endometrial carci-noma must be considered, and endometrial sampling
obtained, when atypical endometrial cells or atypical glan-dular cells of
undetermined significance (AGUS) are found on the Pap smear.
The most
common indication for endometrial sampling is abnormal bleeding. After
ruling out pregnancy in pre-menopausal women, an adequate tissue sample can be
obtained with relatively little discomfort. Further man-agement is usually
dictated by the results of the biopsy specimen. Dilatation and curettage (D&C) or hys-teroscopy with directed endometrial biopsy may beundertaken
when outpatient sampling is not possible (e.g., because of a stenotic cervical
os or a patient who cannot tolerate the outpatient procedure) or when the
outpatient sampling has been nondiagnostic.
Sometimes the office endometrial
biopsy will be reported as having “insufficient tissue for diagnosis.” In a
postmenopausal woman who is not taking hormone therapy, this result is
compatible with an atrophic condition of the endometrium. In other cases, the
clinical suspicion of a pos-sible hyperplastic endometrial process may be high
enough to warrant hysteroscopic evaluation with directed sampling, which allows
more complete evaluation of the endometrium as well as direct diagnosis of
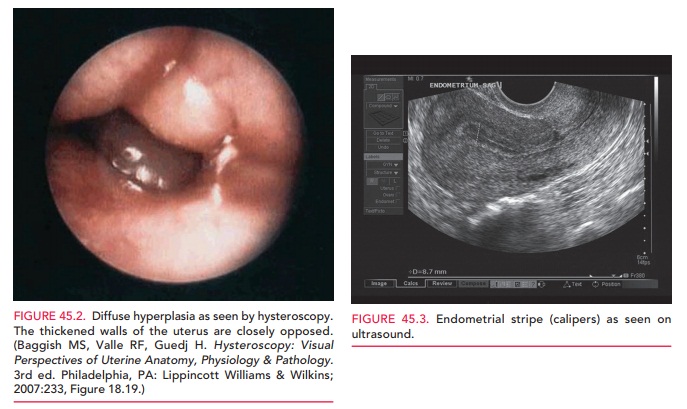
polyps, myomas, and structural abnormalities (Fig. 45.2).
Transvaginal
ultrasound (with or without the instal-lation of fluid for
contrast, sonohysterography) may be used as an adjunct means of evaluation for
endometrial hyperplasia as well as for polyps, myomas, and structural
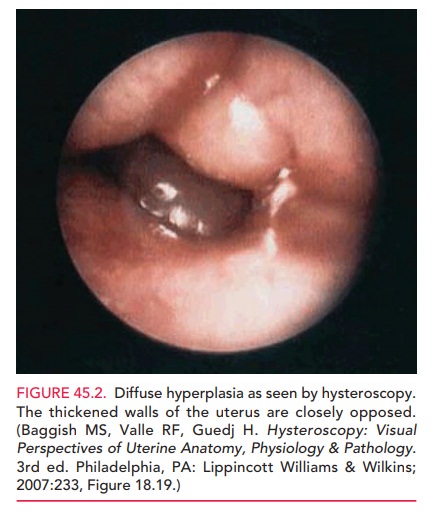
abnormalities of the uterus. An endometrial thickness of >5 mm in a
postmenopausal patient, a polypoid mass, or fluid collection is often
considered an indication for fur-ther evaluation and histologic samples. It is
also useful in patients who have multiple medical problems, to help determine
if the risks of endometrial sampling are less than the risk of not sampling. Nevertheless, an endometrialstripe of less
than 5 mm, although consistent with menopause and endometrial atrophy, does not
exclude the possibility of a nonestrogen-dependent carcinoma of the atrophic endometrium
(Fig. 47.3). The value of transvaginal ultrasonography in a premenopausal
woman is less significant, given day-to-day variations throughout menstrual
cycle.
In women with breast cancer treated with tamoxifen, the optimal manner of monitoring the endometrium is unclear. Tamoxifen acts as a weak estrogen and is associatedwith increased risk of endometrial hyperplasia and carcinoma. Most agree that routine ultrasonography and endome-trial biopsy in asymptomatic women are not necessary. Endometrial abnormalities should be excluded in the presence of new symptoms, such as bloody vaginal dis-charge, spotting, or AUB.
Management
The primary goals of treating
endometrial hyperplasia are to reduce the risk of malignant transformation and
to control the presenting symptoms. Synthetic
progesterones orother progestins are central in the medical treatment of
endome-trial hyperplasia. They act through a number of pathways.First, they
work to alter the enzymatic pathways, which eventually convert endogenous
estradiol to weaker es-trogens. Secondly, they decrease the number of estrogen
Finally, the stimulation of progesterone receptors results in thin-ning of the
endometrium and stromal decidualization. With time, this results in a decrease
in endometrial glandu-lar proliferation, which renders the endometrium
atrophic.
In cases of hyperplasia without
atypia, medical ther-apy is first utilized. The mean duration of progression
from endometrial hyperplasia to carcinoma in those that do
The most com-mon treatment regimen is cyclic medroxyprogesterone acetate, or
MPA, which is administered for 10 to 14 days each month for at least 3 to 6
months. Continuous admin-istration is equally effective and may aid with
compliance in patients who have an irregular cycle length.
Many view hyperplasia with atypia
as a continuum with endometrial cancer. Hence, aggressive therapy for these
patients is warranted, given the increased likelihood of pro-gression to
endometrial cancer. After initial
diagnosis, D&Cis indicated to
better sample the endometrium and exclude under-lying endometrial cancer. In
young women who desire futurefertility, long-term, high-dose progestin therapy
may be used in an attempt to avoid a hysterectomy. As an alterna-tive to oral
therapy, the progesterone intrauterine contra-ceptive has been reported to have
response rates ranging from 58% to 100%. Definitive therapy by hysterectomy is
recommended after completing childbearing. Patients who are treated medically
for atypical hyperplasia should also be followed with periodic endometrial
sampling (every 3 months after therapy), so treatment response can be
monitored.
Related Topics