Chapter: Obstetrics and Gynecology: Cancer of the Uterine Corpus
Endometrial Cancer
ENDOMETRIAL CANCER
Endometrial carcinoma is
typically a disease of post-menopausal women. Between 15% and 25% of postmenopausalwomen with bleeding have
endometrial cancer. A majority ofcases are diagnosed while in stage I
(72%). Despite recog-nition at early stages, endometrial cancer is the eighth
leading site of cancer-related mortality among women in the United States.
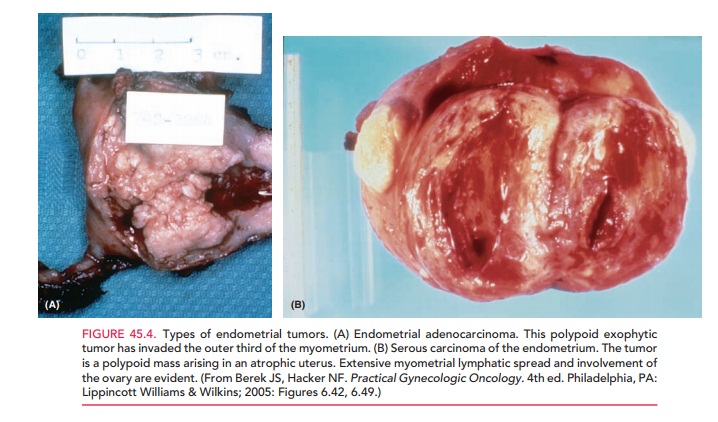
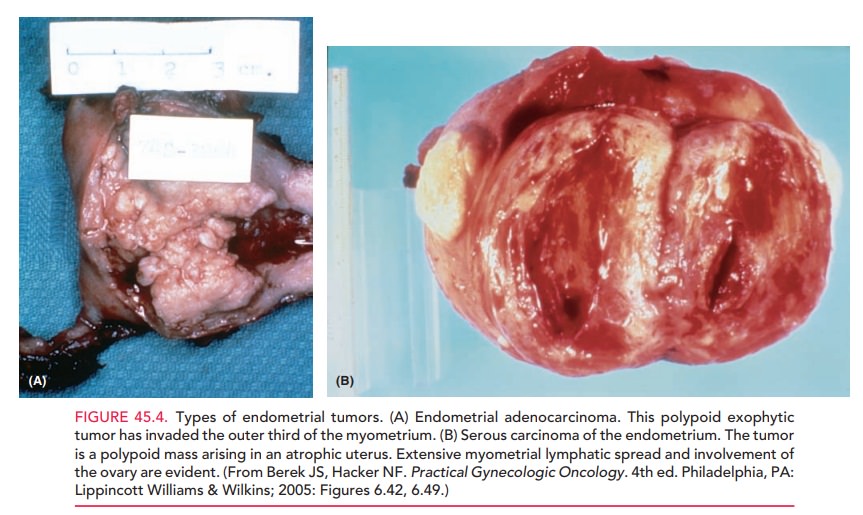
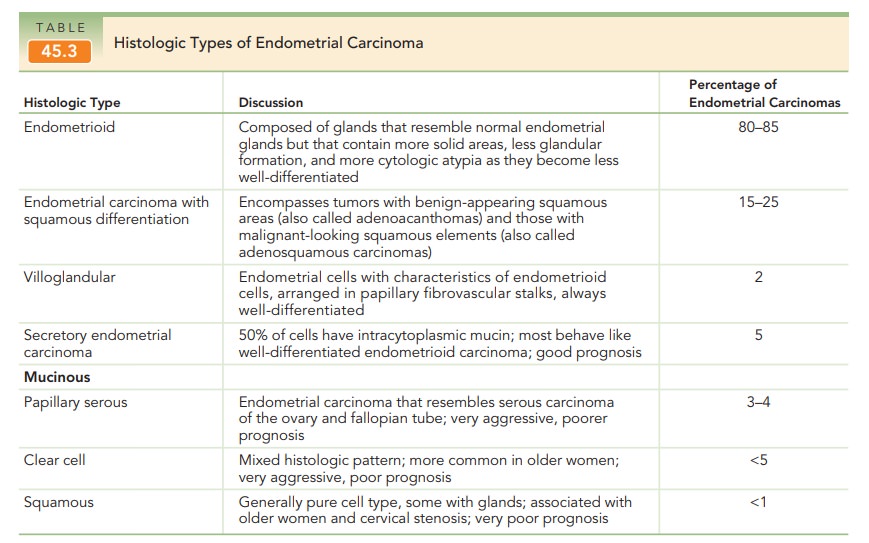
Most primary endometrial carcinomas are adenocar-cinomas (Fig. 45.4).
Because squamous epithelium may coexist with the glandular
elements in an adenocarcinoma, descriptive terms that include the squamous
element may be used. In cases where the squamous element is benign and makes up
less than 10% of the histologic picture, the term adenoacanthoma is used. Uncommonly, the squamous ele-ment may
appear malignant on histologic assessment and is then referred to as adenosquamous carcinoma. Other
descriptions, such as clear cell
carcinoma and papillaryserous
adenocarcinoma, may be applied, depending on thehistologic architecture.
All of these carcinomas are consid-ered under the general category of
adenocarcinoma of the endometrium and are treated in a similar manner.
Pathogenesis and Risk Factors
Two kinds of endometrial carcinoma
have been identified. Type I endometrial carcinoma is “estrogen-dependent” and accounts for approximately 90% of cases. It
is most commonly caused by an excess of estrogen unopposed by progestins. These
cancers tend to have low-grade nuclear atypia, endometrioid cell types, and an
overall favorable prognosis. The second type, Type II or “estrogen-independent” endometrial carcinoma, occurs sponta-neously,
characteristically in thin, older postmenopausal women without unopposed
estrogen excess, arising in an atrophic endometrium rather than a hyperplastic
one. These cancers tend to be less well-differentiated, with a
Estrogen-independent cancer is less com-mon than estrogen-dependent cancer.
Unusual histologic subtypes, including papillary serous adenocarcinoma and
clear cell adenocarcinoma of the endometrium, tend to be more aggressive than
the more common adenocarcinoma (Table 45.3).
Endometrial
carcinoma usually spreads throughout the endometrial cavity first and then
begins to invade the myometrium, endocervical canal, and eventually the
lymphatics. Hematogenous spread occurs with endometrial
carcinoma more readily than in cervical cancer or ovarian cancer. Invasion of
adnexal structures may occur through lymphat-ics or direct implantation through
the fallopian tubes. After extrauterine spread to the peritoneal cavity, cancer
cells may spread widely in a fashion similar to that of ovarian cancer.
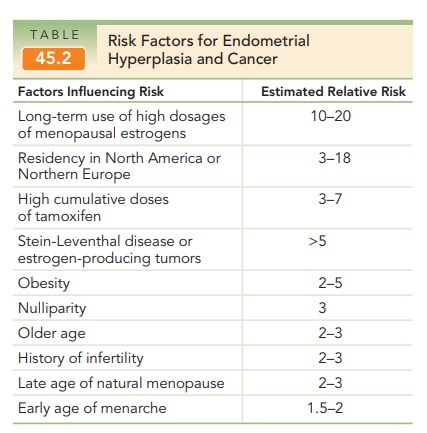
As mentioned earlier, the risk
factors for developing endometrial cancer are identical to those for
endometrial hyperplasia (see Table 45.2).
Diagnosis
Endometrial
sampling, prompted by vaginal bleeding, most frequently establishes the
diagnosis of endometrial cancer. Vaginal bleeding or discharge is
the only presenting complaint in 80% to 90% of women with endometrial
carcinoma. In some, often older patients, cervical ste-nosis may sequester the
blood in the uterus, with the presentation being hematometra or pyometra and a
puru-lent vaginal discharge. In more advanced disease, pelvic dis-comfort or an
associated sensation of pressure caused by uterine enlargement or extrauterine
disease spread may accompany the complaint of vaginal bleeding or even be the
presenting complaint. Fewer than 5% of
women found to haveendometrial carcinoma are actually asymptomatic.
Special
consideration should be given to the patient who pre-sents with postmenopausal bleeding (i.e., bleeding
that occurs after 6 months of amenorrhea in a patient who has been diagnosed as
menopausal).
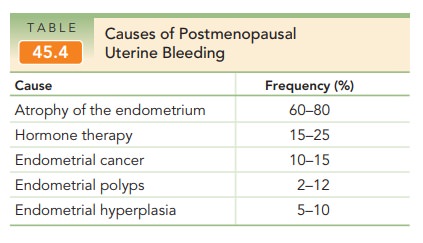
In this group of patients, it is
mandatory to assess the endometrium histologically because the risk of
endometrial carcinoma is approximately 10% to 15%, although other causes are
more common (Table 45.4). Other gynecologic assessments should also be made,
including careful physical and pelvic examination, as well as a screening Pap
smear. Preoperative measurement of the CA
125 level may be appropri-ate, because it is frequently elevated in women with
advanced-stage disease. Elevated levels of CA 125 may assist in
predictingtreatment response or in posttreatment surveillance.
Prognostic Factors
The current International
Federation of Gynecology and Obstetrics (FIGO) staging of endometrial cancer
(adopted in 1988) lists three grades of endometrial carcinoma:
·
G1 is well-differentiated
adenomatous carcinoma (less than 5% of the tumor shows a solid growth pattern).
·
G2 is moderately differentiated
adenomatous carcinoma with partly solid areas (6% to 50% of the tumor shows a
solid growth pattern).
·
G3 is poorly differentiated or
undifferentiated (greater than 50% of the tumor shows a solid growth pattern).
Most patients with endometrial
carcinoma have G1 or G2 lesions by this classification, with 15% to 20% having
undifferentiated or poorly differentiated G3 lesions.
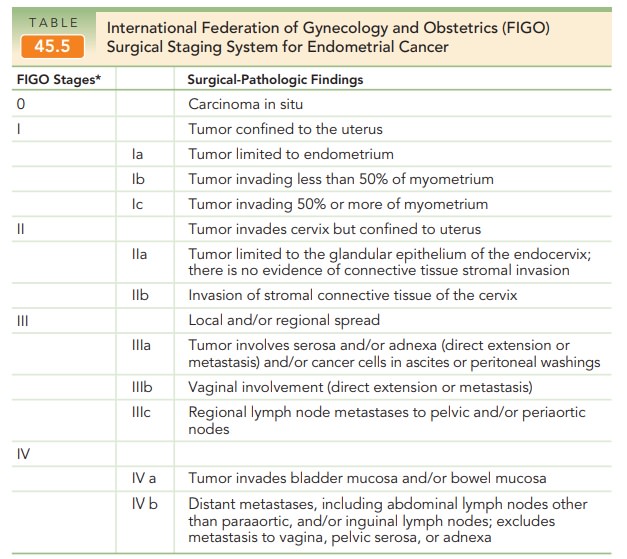
The FIGO staging system
incorporates elements correlated with prognosis and risk of recurrent disease—
histologic grade, nuclear grade, depth of myometrial inva-sion, cervical
glandular or stromal invasion, vaginal and adnexal metastasis, cytology status,
disease in the pelvic and/or paraaortic lymph nodes, and presence of distant
metastases (Table 45.5). The single most important prog-nostic factor for
endometrial carcinoma is histologicgrade.
Histologically, poorly differentiated or undiffer-entiated tumors are
associated with a considerably poorer prognosis because of the likelihood of
extrauterine spread through adjacent lymphatic and peritoneal fluid. Depthof myometrial invasion is the
second most importantprognostic factor.
Survival
rates vary widely, depending on the grade of tumor and depth of penetration
into the myometrium. A patient with aG1 tumor that
does not invade the myometrium has a 95% 5-year survival rate, whereas a
patient with a poorly differ-entiated (G3) tumor with deep myometrial invasion
may have a 5-year survival rate of only 20%.
Treatment
Hysterectomy
is the primary treatment of endometrial cancer. The addition of complete
surgical staging with an assessment of retroperitoneal lymph nodes is not only
therapeutic, but also is associated with improved survival.
Complete surgical staging
includes pelvic washings, bilat-eral pelvic and paraaortic lymphadenectomy, and
com-plete resection of all disease. Sampling of the common iliac nodes,
regardless of depth of penetration or histo-logic grade, may provide further
information about the histologic grade and depth of invasion. Palpation of
lymph nodes is equally inaccurate and should not substitute sur-gical resection
of nodal tissue for histopathology.
Exceptions to the need for
surgical staging include young or perimenopausal women with grade 1
endometri-oid adenocarcinoma associated with atypical endometrial hyperplasia,
and women at increased risk for mortality secondary to comorbidities. Women in
the former group who desire to maintain their fertility may be treated with
high-dose progestin monitored by serial endometrioid sampling. Women in the
latter group may be treated with vaginal hysterectomy. In ultra-high-risk
surgical patients, therapeutic radiation may be used as primary treatment,
although results are suboptimal.
Postoperative radiation therapy should be tailored to known metastatic disease or used in cases of recurrence. In patients with surgical stage I disease, radiation therapy may reduce the risk of recurrence, but does not improve survival. For women with positive lymph nodes (stage IIIc) disease, radia-tion therapy is critical in improving survival rates. Women withintraperitoneal disease are treated with surgery, followed by systemic chemotherapy or radiation therapy or both.
Recurrent Endometrial Carcinoma
Postoperative surveillance for
women who have not received radiation therapy involves speculum and
recto-vaginal examinations every 3 to 4 months for 2 to 3 years, and then twice
a year to detect pelvic recurrent disease, particularly in the vagina. Women who have received radia-tion therapy
have a decreased risk of vaginal recurrence as well as fewer therapeutic
options to treat recurrence. Therefore, these women benefit less from frequent
surveillance with cervical cytology screening and pelvic examinations for
detection of recurrent disease.
Recurrent endometrial carcinoma
occurs in about 25% of patients treated for early disease, one-half within 2
years, and three-fourths within 3 to 4 years. In general, those with recurrent
vaginal disease have a better prog-nosis than those with pelvic recurrence, who
in turn fare better than those with distant metastatic disease (lung, abdomen,
lymph nodes, liver, brain, and bone).
Recurrent
estrogen-dependent or progestin-dependent cancer may respond to high-dose progestin therapy. A major
advantage of high-dose progestin therapy is its minimal compli-cation rate.
Chemotherapy with doxorubicin, cisplatin, and paclitaxel produces occasional
favorable short-term results, but long-term remissions with these therapies are
rare.
Hormone Therapy after Treatment for Endometrial Carcinoma
The use of estrogen therapy in patients previously treated for endometrial
carcinoma has long been considered con-traindicated because of the concern that
estrogen might activate occult metastatic disease.
Hormone
therapy can be used in the presence of the same indications as for any other
woman, except that the selection of appropriate candidates for estrogen
treatment should be based on prognostic indicators and the patient must be
willing to assume the risk. Cautious individualized
assessment of risks and benefits should therefore be made on a case-by-case
basis.
Related Topics