Chapter: Obstetrics and Gynecology: Cancer of the Uterine Corpus
Endometrial Hyperplasia
ENDOMETRIAL HYPERPLASIA
Endometrial hyperplasia is the
most common precursor to endometrioid adenocarcinoma. In 1994, the World Health
Organization defined a classification system of endome-trial hyperplasia based
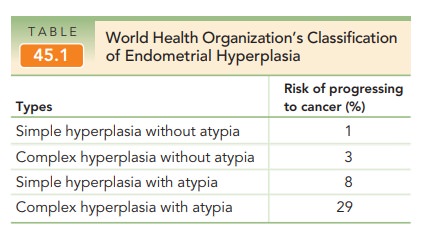
on four types of simple and com-plex hyperplasia, with or without atypia (see Table 45.1).
Types
SIMPLE HYPERPLASIA
Simple
hyperplasia is the least significant form of endome-trial
hyperplasia and is not commonly associated with pro-gression to endometrial
carcinoma. In this type of
hyperplasia,both glandular elements and stromal cell elements proliferate
excessively. Histologically, glands vary markedly in size, fromsmall to
cystically enlarged (the hallmark of this hyper-plasia). Cystic glandular
hyperplasia should not be confused with a normal postmenopausal variant—cystic
involution of the endometrium—which is histologically not a hyper-plastic
condition.
COMPLEX HYPERPLASIA
Complex hyperplasia represents an abnormal
proliferation ofprimarily glandular elements without concomitant proliferation
of stromal elements. This increased gland-to-stroma
ratio givesthe endometrium a “crowded” picture, frequently with glands
appearing almost back-to-back. As the severity of the hyperplasia increases,
the glands become more crowded and more structurally bizarre. It is thought
that complex hyper-plasia represents a true intraepithelial neoplastic process,
and it is occasionally found coexisting with areas of endome-trial
adenocarcinoma.
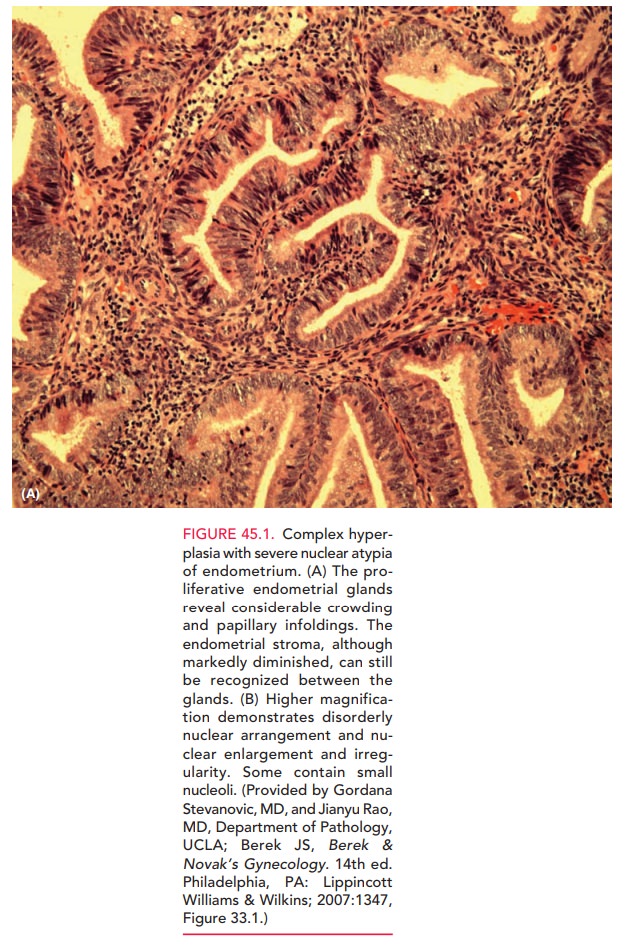
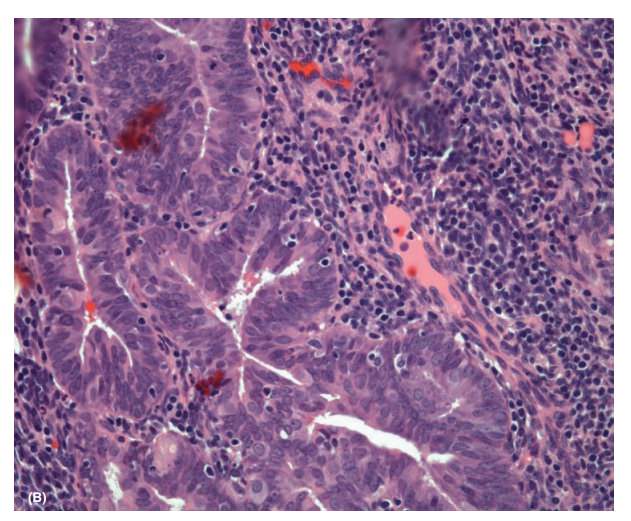
HYPERPLASIA (SIMPLE OR COMPLEX) WITH CYTOLOGICAL ATYPIA
Hyperplasia characterized by
significant numbers of glandu-lar elements that exhibit cytological atypia and disordered maturation (loss of cellular
polarity, nuclear enlargement with increased nucleus-to-cytoplasm ratio, dense
chromatin, and prominent nucleoli), is considered a precursor lesion to
endometrial carcinoma (Fig. 45.1).
Pathophysiology and Risk Factors
The
primary process central to the development of endometrial hyperplasia (and most
endometrial cancer) is overgrowth of the endometrium in response to excess
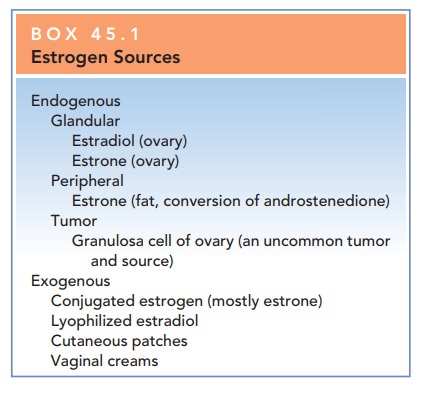
unopposed estrogen. Sourcesof estrogen may be endogenous (ovarian; peripheral
con-version of androgenic precursors) or exogenous
(Box 45.1). Endometrial proliferation represents a normal part of the menstrual
cycle and occurs during the follicular, or estrogen-dominant, phase of the
cycle. With continued estrogen stimulation through either endogenous
mecha-nisms or by exogenous administration, simple endometrial
Research suggests that this transformation may be
time- and dose-dependent. When proliferation becomes hyperplasia is not clear,
although studies showing sequential change suggest it requires 6 months or
longer of stimulation without proges-terone opposition. The risk factors for
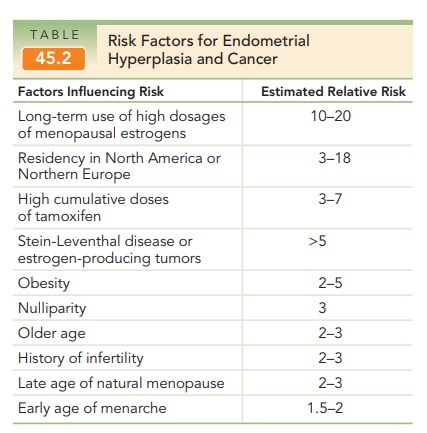
hyperplasia and endometrial cancer are identical (Table 45.2).
The risks of underlying
endometrial cancer following biopsy-proven hyperplasia are as follows: 1% for
simple, 3% for complex, and 8% for simple atypia. Complex atyp-ical hyperplasia
was reported to occur in 29% of cases. Inone
study, more than 42% of women with endometrial atypia had invasive endometrial
cancer demonstrated when hysterec-tomy was performed within 3 months.
Related Topics