Volumetric Analysis | Chemistry Practical Laboratory Experiment - Estimation of oxalic acid | 12th Chemistry : Practicals
Chapter: 12th Chemistry : Practicals
Estimation of oxalic acid
Estimation of
oxalic acid
Aim :
To estimate
the amount of oxalic acid dissolved in 1250 ml of the given unknown solution
volumetrically. For this you are given with a standard solution of HCl solution
of normality 0.1010 N and sodium hydroxide solution as link solution.
Principle:
Neutralization
of Sodium hydroxide by HCl is given below. To indicate the end point,
phenolphthalein is used as an indicator.
NaOH +
HCl → NaCl + H2O
Neutralization
of Sodium hydroxide by oxalic acid is given below. To indicate the end point,
phenolphthalein is used as an indicator.

Short procedure:
Procedure :
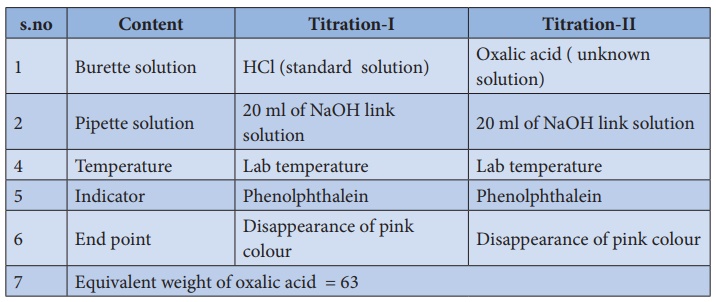
Titration–I
(standard HCl )Vs (link NaOH)
Burette
is washed with water, rinsed with HCl solution and filled with same HCl
solution up to the zero mark. Exactly 20 ml of NaOH is pipetted out into the
clean, washed conical flask. To This solution 2 to 3 drops of phenolphthalein
indicator is added and titrated against HCl solution from the burette. HCl is
added drop wise till the pink colour disappears completely. Burette reading is
noted and the same procedure is repeated to get concordant values.
Titration –I
(standard HCl )Vs (link NaOH)
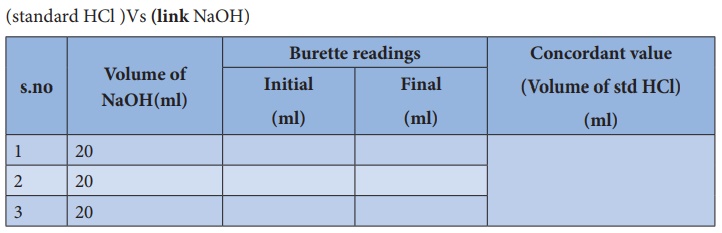
Calculation :
Volume
of NaOH(link) solution V1 = 20 ml
Normality
NaOH(link) solution N1 = ? N
Volume of
standard HCl solution V2 = ml
Normality
of standard HCl solution N2 = 0.1010
N
According to normality equation:
V1
x N1 = V2 x N2
N1 = ? ×
0.1010 / 20 =
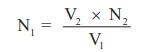
Normality
NaOH (link) solution N1 = ------------X---------N
Titration–II
(Unknown oxalic acid ) Vs (Link
NaOH)
Burette
is washed with water, rinsed with oxalic acid solution and filled with same
oxalic acid solution up to the zero mark. Exactly 20 ml of NaOH solution is
pipetted out into the clean, washed conical flask. To This solution 2 to 3
drops of phenolphthalein indicator is added and titrated against oxalic acid
solution from the burette. oxalic acid is added drop wise till the pink colour
disappears completely. Burette reading is noted and the same procedure is
repeated to get concordant values.
Titration –II
(Link NaOH )Vs (Unknown oxalic acid solution)
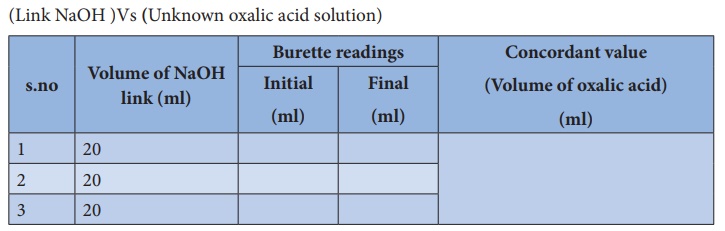
Calculation :
Volume of
Unknown oxalic acid solution V1 = ml
Normality
of Unknown oxalic acid solution N1 = ? N
Volume
of NaOH solution V2 = 20 ml
Normality
NaOH solution N2 = N
According
to normality equation:
V1
x N1 = V2 x N2
N1
= V2 x N2 / V1
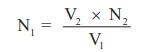
Normality
of Unknown oxalic acid solution N1 =
_____Y__________N
Weight calculation:
The
amount of oxalic acid dissolved in 1 lit of the solution = (Normality) x
(equivalent weight)
The
amount of oxalic acid dissolved in 1250 ml of the solution = Normality x
equivalentweight x 1250 / 1000
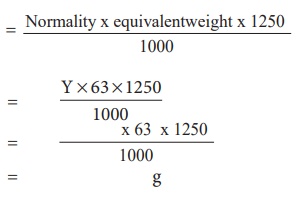
Report :
The
amount of oxalic acid dissolved in 1250 ml of the solution = g
Related Topics