Volumetric Analysis | Chemistry Practical Laboratory Experiment - Estimation of oxalic acid | 12th Chemistry : Practicals
Chapter: 12th Chemistry : Practicals
Estimation of oxalic acid
Estimation of
oxalic acid
Aim :
To
estimate the amount of oxalic acid dissolved in 500 ml of the given solution
volumetrically. For this you are given with a standard solution of ferrous
ammonium sulphate (FAS) of normality 0.1 N and potassium permanganate solution
as link solution.
Principle:
During
these titrations, oxalic acid is oxidized to CO2 and MnO4-
ions (from KMnO4 ) is reduced to Mn2+ ion.
Oxidation : MnO − + 8H+ + 5e− → Mn2+ + 4H O
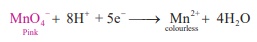
Reduction : 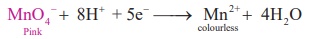
Overall reaction
5(COOH)2
+ 2MnO4 −
+ 6H+ → 10CO2 + 2Mn2+ + 8H2O
Since one
mole oxalic acid releases 2 moles of electrons, the equivalent weight of oxalic
acid = 106/2 = 63 (oxalic acid is dihydrated)
Short procedure:
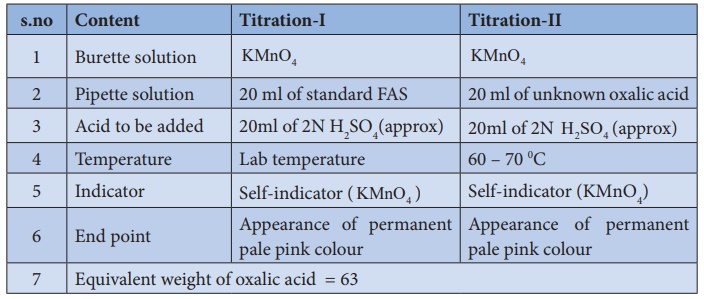
Procedure :
Titration–I
(Link
KMnO4)Vs (Standard FAS )
Burette
is washed with water, rinsed with KMnO4 solution and filled with
same KMnO4 solution up to the zero mark. Exactly 20 ml of standard
FAS solution is pipetted out into the clean, washed conical flask. To this FAS
solution, approximately 20ml of 2N sulphuric acid is added. This mixture is
titrated against KMnO4 Link solution from the burette. KMnO4
is added drop wise till the appearance of permanent pale pink colour. Burette
reading is noted and the same procedure is repeated to get concordant values.
Titration –I
(Link KMnO4 )Vs (Standard FAS solution)
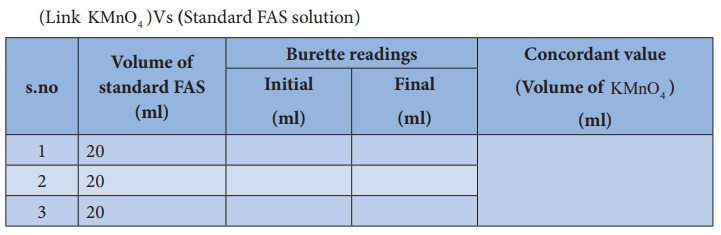
Calculation :
Volume of
KMnO4 (link) solution V1 = ml
Normality
KMnO4 (link) solution N1 = ? N
Volume of
standard FAS solution V2 = 20 ml
Normality
of standard FAS solution N2 = 0.1 N
According
to normality equation:
V1
x N1 = V2 x N2
N1
= V2 x N2 / V1
=
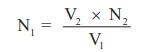
Normality
KMnO4 (link) solution N1 = ____________ N
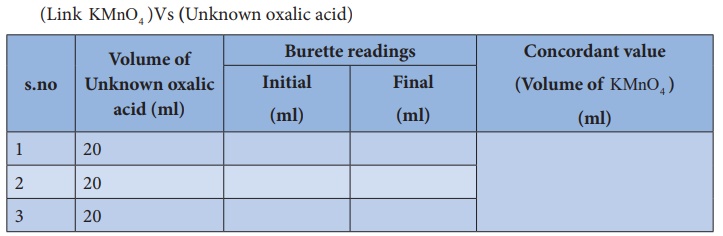
Titration–II
(Unknown oxalic acid ) Vs (Link KMnO4 )
Burette
is washed with water, rinsed with KMnO4 solution and filled with
same KMnO4 solution up to the zero mark. Exactly 20 ml of unknown
oxalic acid solution is pipetted out into the clean, washed conical flask. To
this oxalic acid solution approximately 20ml of 2N sulphuric acid is added.
This mixture is heated to 60 – 700C using Bunsen burner and that hot solution
is titrated against KMnO4 Link solution from the burette. KMnO4
is added drop wise till the appearance of permanent pale pink colour. Burette
reading are noted, the same procedure is repeated to get concordant values.
Titration –II
(Link
KMnO4 )Vs (Unknown oxalic
acid)
Calculation :
Volume of
Unknown oxalic acid solution V1 = 20 ml
Normality
of Unknown oxalic acid solution N1 = ? N
Volume of
KMnO4 (link) solution V2 = ml
Normality
KMnO4 (link) solution N2 = N
According
to normality equation:
V1 x N1 = V2
x N2
N1
= V2 x N2 / V1
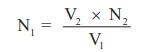
Normality
of Unknown oxalic acid solution N1 = ________ Y _______ N
Weight calculation:
The
amount of oxalic acid dissolved in 1 lit of the solution =(Normality) x (equivalent weight)
The
amount of oxalic acid dissolved in 500 ml of the solution = [Y × 63 × 500] / 1000
= [ ? x
63 x 500] / 1000
= ____ g
Report :
The
amount of oxalic acid dissolved in 500 ml of given the solution = g
Related Topics