Chapter: Modern Medical Toxicology: General Principles: General Management of Poisoning
Elimination - General Management of Poisoning
Elimination
The
various methods of eliminating absorbed poisons from the body include the
following:
·
Forced Diuresis
·
Extracorporeal techniques
o Haemodialysis
o Haemoperfusion
o Peritoneal
dialysis
o Haemofiltration
o Plasmapheresis
o Plasma
perfusion
o Cardiopulmonary
bypass.
Forced Diuresis
Most
drugs taken in overdose are extensively detoxified by the liver to produce
inactive metabolites which are voided in the urine. Sometimes hepatic
degradation produces active metabolites, but the secondary compounds are then
converted to non-toxic derivatives. Under these circumstances, forced diuresis
is inappropriate.
The
procedure should be undertaken only if the following conditions are satisfied:
·
A substantial proportion of the drug
is excreted unchanged.
·
The drug is distributed mainly in
the extracellular fluid.
·
The drug is minimally protein-bound.
·
Principle—
o Most
drugs are weak electrolytes and exist partly as undissociated molecules at
physiological pH. The extent of ionisation is a function of the ionisation
constant of the drug (Ka for both acids and bases), and the pH of the medium in
which it is dissolved. Ionisation constants are usually expressed in the form
of their negative logarithm, pKa. Hence the pKa scale is analogous to the pH
notation : the stronger an acid the lower its pKa, and the stronger a base the
higher its pKa.
o Thus
when pKa = pH, the concentrations of ionised and non-ionised drugs are equal.
Cell membranes are most permeable to
substances that are lipid soluble and in the non-ionised, rather than the
ionised form. Thus the rate of diffusion from the renal tubular lumen back into
the circulation is decreased when a drug is maximally ionised. Because
ionisation of acidic drugs is increased in an alkaline environment, and that of
basic drugs is increased in an acid solu- tion, manipulation of the urinary pH
enhances renal excretion.
Forced alkaline diuresis :
·
This is most useful in the case of
phenobarbitone, lithium, and salicylates.
·
Administer 1500 ml of fluid IV, in
the first hour as follows :
–– 500 ml of 5% dextrose
––
500 ml of 1.2 or 1.4% sodium bicarbonate
––
500 ml of 5% dextrose.
Forced acid diuresis :
Forced
acid diuresis is no longer recommended for any drug or poison, including
amphetamines, strychnine, quinine or phencyclidine
Extracorporeal Techniques
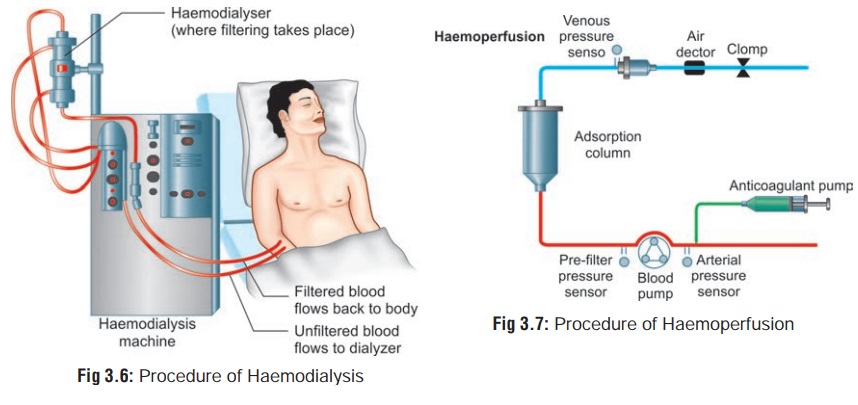
Haemodialysis (Fig 3.6)
·
Haemodialysis was first used in 1913
in experimental poisoning, but was not applied clinically until 1950, when it
was used for the treatment of salicylate overdose. It was widely employed in
the subsequent two decades accompa- nied by much adulatory reportage of its
efficacy in medical journals. However, the popularity of haemodialysis has
declined since then owing to authentic observation of its lack of utility in several
types of poisoning, and the high incidence of complications such as infection,
thrombosis, and air embolism.
·
All drugs are not dialysable, and so
it must be ensured before embarking on this procedure that the following
conditions are satisfied:
o The
substance should be such that it can diffuse easily through a dialysis
membrane.
o A
significant proportion of the substance should be present in plasma water or be
capable of rapid equili- bration with it.
o The
pharmacological effect should be directly related to the blood concentration.
o Table 3.19 outlines
the various factors in a toxin which can affect the outcome of haemodialysis.
Extensive plasma protein binding, insolubility in water, and high molecular
weight are the three most important factors in making haemodialysis
ineffective.

Procedure—
·
The three basic components of
haemodialysis are the blood delivery
system, the dialyser itself, and
the compo- sition and method of delivery
of the dialysate. For acute haemodialysis, catheters are usually placed in
the femoral vein and passed into the inferior venacava. Blood from one is
pumped to the dialyser (usually by a roller pump) through lines that contain equipment
to measure flow and pressure within the system. Blood returns through the
second catheter. Dialysis begins at a blood flow rate of 50 to 100 ml/min, and
is gradually increased to 250 to 300 ml/min, to give maximal clearance.
Indications for haemodialysis—
·
Haemodialysis may be considered in
those patients not responding to standard therapeutic measures while treating a
dialysable toxicant (vide infra). It
may also be considered a part of supportive care whether the toxicant is dialysable or not in the
following situations: Stage 3 or 4 coma, or hyperactivity caused by a
dialys-able agent which cannot be treated by conservative means, marked
hyperosmolality which is not due to easily corrected fluid problems, severe
acid-base distur-bance not responding to therapy, or severe electrolyte
disturbance not responding to therapy.
––
Best indications: Dialysis should be initiated, regardless of clinical
condition, in the following situations: after heavy metal chelation in patients
with renal failure, and following significant ethylene glycol or methanol
ingestion.
––
Very good indications: Dialysis is usually effec-tive in patients with severe
intoxications with the following agents:
-- Lithium
--
Phenobarbitone -- Salicylates
-- Theophylline.
–– Fairly good indications: Dialysis
may be initiated following exposure to the following agents, if clinical
condition deems the procedure necessary (patient deteriorating despite intense
supportive care):
--
Alcohols
-- Amphetamines -- Anilines
-- Antibiotics -- Boric acid
-- Barbiturates (short acting) --
Bromides
--
Chlorates
-- Chloral hydrate – Iodides
--Isoniazid![]()
--Meprobamate
-- Paraldehyde
-- Fluorides
-- Quinidine
-- Quinin
-- Strychnine
-- Thiocyanates.
–– Poor indications: Dialysis can be
considered as a supportive measure in the presence of renal failure, following
exposure to:
--
Paracetamol
-- Antidepressants -- Antihistamines
-- Belladonna alkaloids --
Benzodiazepines
-- Digitalis and related glycosides
-- Glutethimide
--
Opiates
-- Methaqualone -- Phenothiazines
--
Synthetic anticholinergics.
Complications—
·
Infection (especially AIDS, hepatitis B)
·
Thrombosis
·
Hypotension
·
Air embolism
·
Bleeding (due to use of heparin as a systemic antico-
agulant).
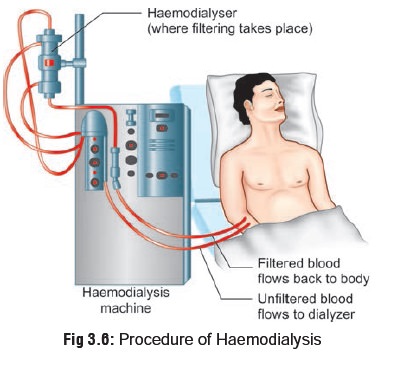
Haemoperfusion (Fig 3.7)
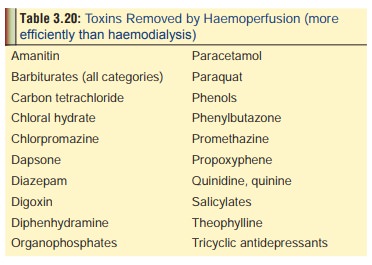
This
is a technique that is increasingly becoming popular since it is capable of
removing many of the toxins that are not removed well by haemodialysis (Table 3.20).
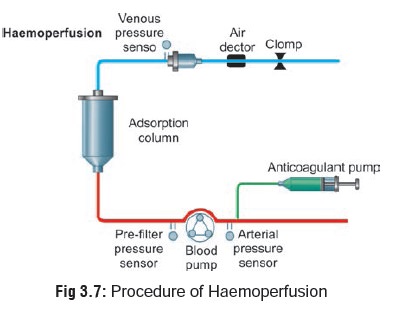
Procedure—
·
An arteriovenous shunt or a double-
lumen venous catheter is inserted into the patient’s vascular tree. The
haemoperfusion column and lines are primed with heparinised saline in
accordance with the manu-facturer’s instructions and connected to the shunt or
catheter. On commencement of perfusion, a bolus of heparin is injected into the
arterial line and heparini-sation is continued by administering an infusion of
heparinised saline.
Complications—
·
Bleeding (because of heparinisation)
·
Air embolism
·
Infection
·
Thrombocytopenia
·
Hypocalcaemia
·
Hypotension.
Peritoneal Dialysis
Although
widely available, peritoneal dialysis today is almost never recommended for
detoxification. In general, it is only 10 to 25% as effective as haemodialysis,
and often only slightly more effective than forced diuresis. It is also time
consuming, requiring 24 hours for successful comple-tion as compared to the 2
to 4 hour cycles of haemodialysis and haemoperfusion. The only advantages are
that it does not require anticoagulation and uses minimal equipment.
Procedure—
·
Peritoneal dialysis works on the
same principle as haemodialysis, allowing the diffusion of toxins from
mesenteric capillaries across the peritoneal membrane into the dialysate
dwelling in the peritoneal cavity. It involves the placing of a stylet catheter
at the bedside under local anaesthesia, or the surgical insertion of a
Tenckhoff catheter in the abdomen. Dialysate fluid is instilled, and 1 to 2
litres is exchanged each hour.
Complications—
·
Pain
·
Haemorrhage (from vascular
laceration)
·
Perforation of viscus
·
Bacterial peritonitis
·
Arrhythmias
·
Volume depletion/overload
·
Pneumonia
·
Pleural effusion
·
Hyperglycaemia
·
Electrolyte imbalance.
Haemofiltration
Haemofiltration is performed similar
to haemodialysis except that the blood is pumped through a haemofilter. An
arte-riovenous pressure difference induces a convective transport of solutes
through a hollow fibre flat sheet membrane. This allows a substantial flow of
plasma water, and a high permeability to compounds with molecular weight less
than 40,000. The procedure can be done intermittently at high ultrafiltrate
rates of upto 6 litres/hour, or continuously at rates of 100 ml/hour (Continuous Arteriovenous Haemofiltration,
or CAVH). The latter is preferred in the treatment of poisoning.
The main advantage of
haemofiltration is that it can remove compounds of large relative molecular
weight (4,500–40,000). Such compounds include aminoglycoside antibiotics and
metal chelates (such as iron-desferrioxamine). CAVH is also useful in poisoning
with lithium, methanol, ethanol, and ethylene glycol.
Haemodiafiltration
This
is a combination of haemofiltration with haemodi- alysis. It has been
undertaken very rarely, and nothing much is known as to its actual advantages,
if any.
Plasmapheresis
Plasmapheresis
is a technique of separating cellular blood components from plasma. The cells
are resuspended in either colloids, albumin, or fresh frozen plasma, and then
reinfused. It is very effective in eliminating toxic substances but exacts a
heavy toll: a part of the patient’s plasma proteins are sacrificed in the
process. Plasmapheresis has been used in cases of overdose with theophylline,
carbamazepine, amanita, mercury, hemlock, etc., but serious complications
greatly limit its utility.
Complications—
·
Bleeding disorders: DIC, thrombocytopenia
·
Hypercoagulation: Cerebral thrombosis, pulmonary embolism,
myocardial infarction.
·
Anaphylaxis.
·
Fluid overload: Hypertension, congestive heart failure.
·
Infection.
·
Vessel perforation, air embolism.
·
Dysequilibrium syndrome: Vomiting, hypovolaemia.
·
Citrate toxicity: Paraesthesias, tetany, chills, arrhyth-
mias.
·
Convulsions.
·
Metabolic alkalosis.
Plasma Perfusion
This is a combination of
plasmapheresis and haemoperfu-sion, and has rarely been used in poisoning.
Cardiopulmonary Bypass
This
is another rarely used experimental procedure in the treatment of poisoning,
and has been shown to be useful in certain cases of overdose involving cardiac
depressants such as verapamil and lidocaine.
Related Topics