Chapter: Clinical Anesthesiology: Anesthetic Management: Renal Physiology & Anesthesia
Effects of Anesthesia & Surgery on Renal Function
Effects of Anesthesia & Surgery on Renal Function
Acute kidney injury (AKI) is a common
periopera-tive problem. It occurs in 1–5% of all hospitalized patients and is a
major contributor to increased hos-pital length of stay, markedly increasing
morbidity, mortality, and cost of care. Patients may develop AKI and kidney
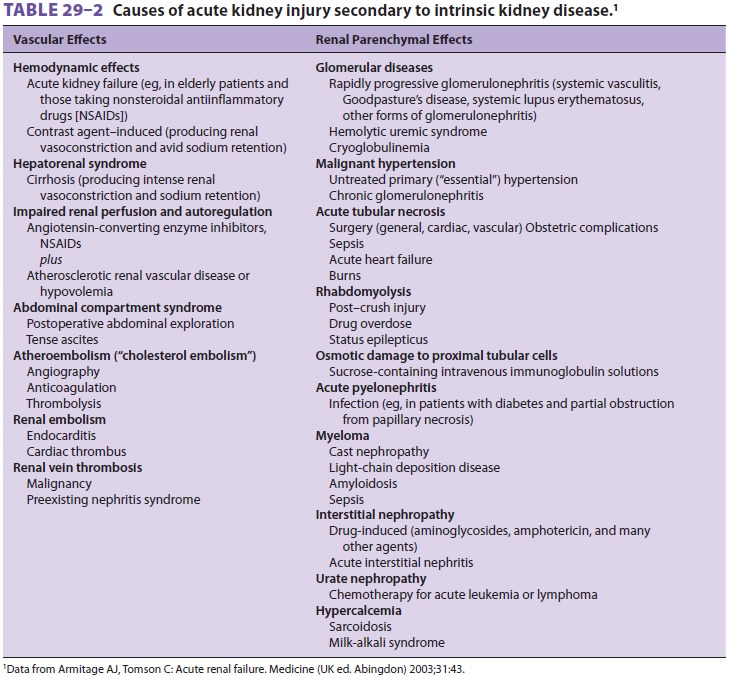
failure secondary to intrinsic kid-ney disease (Table29–2). Risk factors for AKI in the
perioperative setting include preexisting renal impairment, diabetes mellitus,
cardiovascular dis-ease, hypovolemia, and use of potentially nephro-toxic medication
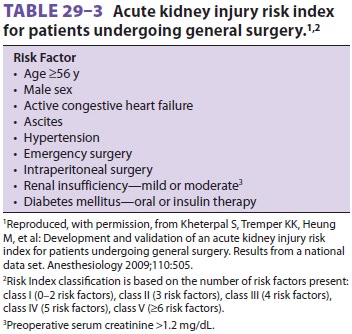
by elderly patients. The risk index in Table29–3 identifies preoperative predictors of AKI following
general surgery.
Clinical studies attempting to define the effects of anesthetic
agents on renal function are compli-cated and difficult. However, several
conclusions can be stated:
·
Reversible decreases
in RBF, GFR, urinaryflow, and sodium excretion occur during both regional and
general anesthesia.
·
Such changes are
usually less pronounced during regional anesthesia.
·
Most of these changes
are indirect and are mediated by autonomic and hormonal responses to surgery
and anesthesia.
·
AKI
is less likely when an adequate intravascular volume and a normal blood
pressure are maintained.
· There is no evidence that currently utilized vapor anesthetic agents cause AKI in patients. However, several studies have reported that compound A, a breakdown product of sevoflurane, produces renal toxicity when administered at low flow rates in laboratoryanimals.
INDIRECT EFFECTS
Cardiovascular
Most inhalation and intravenous
anesthetics pro-duce concentration-dependent cardiac depression or
vasodilation; therefore they are capable of decreas-ing systemic blood
pressure. Depending on the level of sympathetic blockade, spinal or epidural
anes-thesia may cause a drop in systemic blood pressure secondary to decreased
cardiac output as a result of decreased sympathetic tone. This leads to
increased pooling of blood and decreased systemic vascular resistance,
decreased heart rate, and decreased car-diac output. Decreases in blood
pressure below the limits of autoregulation reduce RBF, GFR, urinary flow, and
sodium excretion, and this adverse impact on renal function can be reversed by
administration of pressor agents and intravenous fluids.
Neurologic
Increased sympathetic tone commonly
occurs in the perioperative period as a result of anxiety, pain, light
anesthesia, and surgical stimulation. Heightened sympathetic activity increases
renal vascular resis-tance and activates several hormonal systems , reducing
RBF, GFR, and urine output.
Endocrine
Endocrine changes during sedation and
general anesthesia are a component of the stress response induced by factors
that may include anxiety, pain, surgical stimulation, circulatory depression,
hypoxia, acidosis, and hypothermia. Increases in epinephrine and
norepinephrine, renin, angiotensin II, aldosterone, ADH, adrenocortico-tropic
hormone, and cortisol are common. Cate-cholamines, ADH, and angiotensin II all
reduce RBF by inducing renal arterial constriction. Aldosterone enhances sodium
reabsorption in the distal tubule and collecting tubule, resulting in sodium
retention and expansion of the extracellular fluid compart-ment. Nonosmotic
release of ADH also favors water retention and may result in hyponatremia. The endocrine response to surgery and
anesthesia is at least partly responsible for transient fluidretention seen postoperatively in many
patients.
DIRECT ANESTHETIC EFFECTS
The direct effects of anesthetics on renal function are minor
compared with the secondary effects described above.
Volatile Agents
Halothane, sevoflurane, desflurane, and
isoflurane decrease renal
vascular resistance. As previ-ously noted, compound A, a breakdown product of sevoflurane, has been shown to
cause renaldamage in laboratory animals. Its accumulation
in the breathing circuit is favored by low flow rates. No clinical study has
detected significant renal injury in humans during sevoflurane anesthesia;
nonetheless, some regulatory authorities recommend fresh gas flow of at least 2
L/min with sevoflurane to prevent this theoretical problem.
Intravenous Agents
Opioids and propofol exhibit minor, if
any, effects on the kidney when used alone. Ketamine mini-mally affects renal
function and may, relative to other anesthetic agents, preserve renal function
during hemorrhagic hypovolemia. Agents with α-adrenergic blocking activity may
prevent cate-cholamine-induced redistribution of RBF. Drugs with
antidopaminergic activity—such as metoclo-pramide, phenothiazines, and
droperidol—may impair the renal response to dopamine. Inhibition of
prostaglandin synthesis by NSAIDs such as ketoro-lac prevents renal production
of vasodilatory pros-taglandins in patients with high levels of angiotensinand norepinephrine; attenuation of prostaglandin synthesis in
this setting may result in AKI. ACE inhibitors block the protective effects of
angioten-sin II and may result in reductions in GFR during anesthesia.
Other Drugs
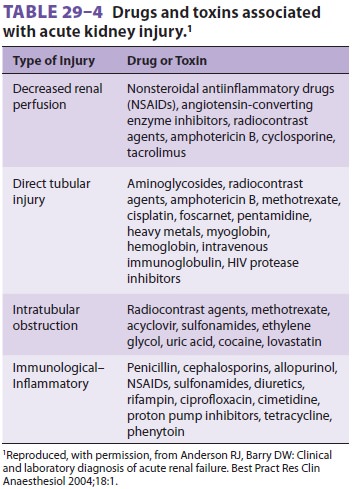
Many medications, including
radiocontrast agents, used in the perioperative period can adversely affect
renal function, particularly in the setting of preex-isting renal dysfunction (Table29–4).
Mechanisms of injury include vasoconstriction, direct tubular injury,
drug-induced immunological and inflamma-tory responses, and renal microvascular
or tubular obstruction. In addition to intravenous hydration, pretreatment with
N-acetylcysteine (600 mg orally every
12 h in four doses beginning prior to contrast administration) has been shown
to decrease the risk of radiocontrast agent–induced AKI in patients with
preexisting renal dysfunction. N-Acetylcysteine’s
protective action may be due to its free radical scavenging or sulfhydryl donor
(reducing) prop-erties. Fenoldopam, mannitol, loop diuretics, and low-dose
dopamine infusion do not help maintain
renal function or confer protection
against AKI, and N-acetylcysteine has
not been shown to be protec-tive in the perioperative setting except in
patients who receive radiocontrast dyes.
DIRECT SURGICAL EFFECTS
In addition to the physiological changes
associated with the neuroendocrine stress response to surgery, certain surgical
procedures can significantly alter renal physiology. The pneumoperitoneum
produced during laparoscopy creates anabdominal compartment
syndrome–like state. The increase in intraabdominal pressure typically
pro-duces oliguria (or anuria) that is generally propor-tional to the
insufflation pressures. Mechanisms include central venous compression (renal
vein and vena cava); renal parenchymal compression; decreased cardiac output;
and increases in plasma levels of renin, aldosterone, and ADH. Abdominal
compartment syndrome can also be produced bysevere intraabdominal tissue edema,
with a similar adverse impact on renal function via the same mechanisms.
Other surgical procedures that can signifi-cantly impair renal
function include cardiopulmo-nary bypass , cross-clamping of the aorta , and
dissection near the renal arteries .
Related Topics