Chapter: Clinical Anesthesiology: Anesthetic Management: Renal Physiology & Anesthesia
Anesthesia: Diuretics
Diuretics
Diuretics increase urinary output by decreasing the reabsorption of Na+ and water. Although clas-sified according to their mechanism of action, many diuretics have more than one such mechanism; hence this classification system is imperfect. Only major mechanisms will be reviewed here.The majority of diuretics exert their action on the luminal cell membrane from within the renal tubules. Because nearly all diuretics are highly protein bound, relatively little of the free drug enters the tubules by filtration. Most diuretics must therefore be secreted by the proximal tubule (usually via the organic anion pump) to exert their action. Impaired delivery into the renal tubules accounts for resistance to diuretics in patients with impaired renal function.
OSMOTIC DIURETICS MANNITOL
Osmotically active diuretics are filtered at the glom-erulus and undergo limited or no reabsorption in the proximal tubule. Their presence in the proximal tubule limits the passive water reabsorption that nor-mally follows active sodium reabsorption. Although their major effect is to increase water excretion, in large doses, osmotically active diuretics also increase electrolyte (sodium and potassium) excretion. The same mechanism also impairs water and solute reab-sorption in the loop of Henle.
Mannitol is the most commonly used osmotic diuretic. It is a six-carbon sugar that normally undergoes little or no reabsorption. In additionto its diuretic effect, mannitol appears to increase RBF. The latter can wash out some of the medullary hypertonicity and interfere with renal concentrat-ing ability. Mannitol appears to activate the intrare-nal synthesis of vasodilating prostaglandins. It also appears to be a free radical scavenger.
Uses
A. Prophylaxis Against Acute Kidney Injury in High-Risk Patients
Many clinicians continue to administer mannitol for renal protection and, less frequently, to convert oliguric acute kidney failure to nonoliguric kidney failure, with the goal of lowering associated mor-bidity and mortality. However, there is no evidence that such use of mannitol provides renal protec-tion, lessens the severity of AKI, or lessens the morbidity or mortality associated with AKI when compared with correction of hypovolemia and preservation of adequate renal perfusion alone. In addition, high-dose mannitol can be nephrotoxic, especially in patients with renal insufficiency.
B. Evaluation of Acute Oliguria
Mannitol will augment urinary output in the set-ting of hypovolemia but will have little effect in the presence of severe glomerular or tubular injury. However, the optimal initial approach to evaluation of acute oliguria is to correct hypovolemia and opti-mize cardiac output and renal perfusion.
C. Acute Reduction of Intracranial Pressure & Cerebral Edema
D. Acute Reduction of Intraocular Pressure in the Perioperative Period
Intravenous Dosage
The intravenous dose for mannitol is 0.25–1 g/kg.
Side Effects
Mannitol solutions are hypertonic and acutely raise plasma and extracellular osmolality. A rapid intra-cellular to extracellular shift of water can transiently increase intravascular volume and precipitatecardiac decompensation and pulmonary edema in patients with limited cardiac reserve. Transient hyponatremia and reductions in hemoglobin con-centration are also common and represent acute hemodilution resulting from rapid movement of water out of cells; a modest, transient increase in plasma potassium concentration may also be observed. It is also important to note that the ini-tial hyponatremia does not represent hypoosmo-lality but reflects the presence of mannitol . If fluid and electrolyte losses are not replaced following diuresis, mannitol administra-tion can result in hypovolemia, hypokalemia, and hypernatremia. The hypernatremia occurs because water is lost in excess of sodium. As noted above, high-dose mannitol can be nephrotoxic, especially in patients with renal insufficiency.
LOOP DIURETICS
The loop diuretics include furosemide (Lasix), bumetanide (Bumex), ethacrynic acid (Edecrin), and torsemide (Demadex). All loop diuretics inhibit Na+ and Cl − reabsorption in the thick ascending limb. Sodium reabsorption at that site requires that all four sites on the Na +–K+–2Cl− luminal car-rier protein be occupied. Loop diuretics compete with Cl− for its binding site on the carrier protein (see Figure 29–4). With a maximal effect, they can promote excretion of 15–20% of the filtered sodium load. Both urinary concentrating and urinary dilut-ing capacities are impaired. The large amounts of Na+ and Cl − presented to the distal nephron over-whelm its limited reabsorptive capability. The result-ing urine remains hypotonic, probably due to rapid urinary flow rates that prevent equilibration with the hypertonic renal medulla or due to interference with the action of ADH on the collecting tubules. A marked increase in diuresis may occur when a loop diuretic is combined with a thiazide diuretic, espe-cially metolazone.
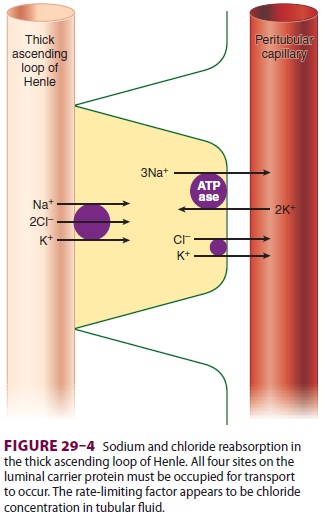
Loop diuretics also increase urinary calcium and magnesium excretion. Ethacrynic acid is the only loop diuretic that is not a sulfonamide deriv-ative, and thus may be the diuretic of choice in patients allergic to sulfonamide drugs. Torsemide may have an antihypertensive action independent of its diuretic effect.
Uses
A. Edematous States (Sodium Overload)
These disorders include heart failure, cirrhosis, the nephrotic syndrome, and renal insufficiency. When given intravenously, these agents can rapidly reverse cardiac and pulmonary manifestations of fluid overload.
B. Hypertension
Loop diuretics may be used as adjuncts to other hypotensive agents, particularly when thiazides (below) alone are ineffective.
C. Evaluation of Acute Oliguria
The response to a small dose (10–20 mg) of furo-semide may be useful in differentiating between oliguria resulting from hypovolemia and oliguria resulting from redistribution of RBF to juxtamed-ullary nephrons. Little or no response is seen with hypovolemia, whereas resumption of normal uri-nary output occurs with the latter. However, the optimal initial approach to evaluation of acute oli-guria is to correct hypovolemia and optimize cardiac output and renal perfusion.
D. Conversion of Oliguric Kidney Failure to Nonoliguric Failure
As with mannitol, discussed earlier, many clinicians continue to administer loop diuretics for renal pro-tectn and to convert oliguric acute kidney failure to nonoliguric kidney failure, despite lack of evi-dence that such use provides renal protection, less-ens the severity of AKI, or lessens the morbidity or mortality associated with AKI, when compared with correction of hypovolemia and preservation of ade-quate renal perfusion alone.
E. Treatment of Hypercalcemia
F. Rapid Correction of Hyponatremia
Intravenous Dosages
The intravenous doses are furosemide, 10–100 mg; bumetanide, 0.5–1 mg; ethacrynic acid, 50–100 mg; and torsemide 10–100 mg.
Side Effects
Increased delivery of Na+ to the distal and collect-ing tubules increases K+ and H+ secretion at those sites and can result in hypokalemia and metabolic alkalosis. Marked Na+ losses will also lead to hypo-volemia and prerenal azotemia; secondary hyper-aldosteronism often accentuates the hypokalemia and metabolic alkalosis. Urinary calcium and magnesium loss promoted by loop diuretics may result in hypocalcemia or hypomagnesemia, or both. Hypercalciuria can result in stone formation. Hyperuricemia may result from increased urate reabsorption and from competitive inhibition of urate secretion in the proximal tubule. Reversible and irreversible hearing loss has been reported with loop diuretics, especially furosemide and ethacrynic acid.
THIAZIDE& THIAZIDE LIKE DIURETICS
This group of agents includes thiazides, containing a benzothiadiazine molecular structure, and also thiazide-like drugs with similar actions but without the benzothiadiazine structure, including chlortha-lidone (Thalitone), quinethazone (Hydromox), metolazone (Zaroxolyn), and indapamide (Lozol). These diuretics act at the distal tubule, including the connecting segment, and inhibition of sodium reabsorption at this site impairs diluting but not concentrating ability. They compete for the Cl − site on the luminal Na+–Cl − carrier protein. When given alone, thiazide and thiazide-like diuretics increase Na+ excretion to only 3–5% of the filtered load because of enhanced compensatory Na+ reab-sorption in the collecting tubules. They also possess carbonic anhydrase–inhibiting activity in the prox-imal tubule, which is usually masked by sodium reabsorption in the loop of Henle and which is probably responsible for the marked diuresis often seen when they are combined with loop diuret-ics. In contrast to their effects on sodium excre-tion, thiazide and thiazide-like diuretics augment Ca2+ reabsorption in the distal tubule. Indapamide has some vasodilating properties and is the only thiazide or thiazide-like diuretic with significant hepatic excretion.
Uses
A. Hypertension
Th iazide and thiazide-like diuretics are often selected as first-line agents in the treatment of hyperten-sion , and they have been shown to improve long-term outcomes in this disorder.
B. Edematous Disorders (Sodium Overload)
Th ese drugs are used to treat mild to moderate edema and congestive heart failure related to mild to moderate sodium overload.
C. Hypercalciuria
Th iazide and thiazide-like diuretics are often used to decrease calcium excretion in patients with hyper-calciuria who form renal stones.
D. Nephrogenic Diabetes Insipidus
The efficacy of these agents in this disorder reflects their ability to impair diluting capacity and increase urine osmolality .
Intravenous Dosages
Th ese agents are only given orally.
Side Effects
Although thiazide and thiazide-like diuretics deliver less sodium to the collecting tubules than loop diuretics, the increase in sodium excretion is enough to enhance K + secretion and frequently results in hypokalemia. Enhanced H+ secretion can also occur, resulting in metabolic alkalosis. Impairment of renal diluting capacity may produce hyponatremia. Hyperuricemia, hyperglycemia, hypercalcemia, and hyperlipidemia may also be seen.
POTASSIUM SPARING DIURETICS
These are weak diuretic agents and characteristically do not increase potassium excretion. Potassium-sparing diuretics inhibit Na+ reabsorption in the col-lecting tubules and therefore can maximally excrete only 1–2% of the filtered Na+ load. They are usually used in conjunction with more potent diuretics for their potassium-sparing effect.
1.Aldosterone Antagonists (Spironolactone & Eplerenone)
Spironolactone (Aldactone) and eplerenone are direct aldosterone receptor antagonists in collecting tubules. They inhibit aldosterone-mediated Na+ reabsorption and K+ secretion. Both agents have been shown to improve survival in patients with chronic heart fail-ure. Aldosterone may produce gynecomastia in male patients due to its antiandrogenic properties.
Uses
These agents may be used as adjuvants in the treat-ment of refractory edematous states associated with secondary hyperaldosteronism . Spironolactone is particularly effective in patients with ascites related to advanced liver disease. They have become part of the standard medical manage-ment of chronic heart failure.
Intravenous Dosage
Th ese agents are only given orally.
Side Effects
These agents can result in hyperkalemia in patients with high potassium intake or renal insufficiency and in those receiving β blockers or ACE inhibitors. Metabolic acidosis may also be seen. Eplerenone lacks spironolactone’s side effects of gynecomastia and sexual dysfunction.
2. Noncompetitive Potassium-Sparing Diuretics
Triamterene (Dyrenium) and amiloride (Midamor) are not dependent on aldosterone activity in the collecting tubule. They inhibit Na+reabsorption and K+ secretion by decreasing the number of open sodium channels in the luminal membrane of col-lecting tubules. Amiloride may also inhibit Na+–K+-ATPase activity in the collecting tubule.
Uses
In patients with hypertension, these agents are often combined with a thiazide or similar diuretic to minimize hypokalemia produced by the other agent. They have been added to more potent loop diuretics in congestive heart failure patients with marked potassium wasting.
Intravenous Dosages
Th ese agents are only given orally.
Side Effects
Amiloride and triamterene can cause hyperkalemia and metabolic acidosis similar to that seen with spi-ronolactone (see above). Both can also cause nau-sea, vomiting, and diarrhea. Amiloride is generally associated with fewer side effects, but paresthesias, depression, muscle weakness, and cramping may occasionally be seen. Triamterene on rare occasions has resulted in renal stones and is potentially neph-rotoxic, particularly when combined with nonste-roidal antiinflammatory agents.
CARBONIC ANHYDRASE INHIBITORS
Carbonic anhydrase inhibitors such as acetazol-amide (Diamox) interfere with Na+ reabsorption and H+ secretion in proximal tubules. They are weak diuretics because the former effect is limited by the reabsorptive capacities of more distal segments of nephrons. Nonetheless, these agents significantly interfere with H+ secretion in the proximal tubule and impair HCO3− reabsorption.
Uses
A. Correction of Metabolic Alkalosis in Edematous Patients
Carbonic anhydrase inhibitors often potentiate the effects of other diuretics.
B. Alkalinization of Urine
Alkalinization enhances urinary excretion of weakly acidic compounds such as uric acid.
C. Reduction of Intraocular Pressure
Inhibition of carbonic anhydrase in the ciliary pro-cesses reduces the formation of aqueous humor and, secondarily, intraocular pressure. Carbonic anhy-drase inhibitors, including oral or intravenous acet-azolamide, oral methazolamide (Neptazane), and ophthalmic topical brinzolamide (Azopt) and dorzol-amide (Trusopt) are often used to treat glaucoma.
Intravenous Dosage
For acetazolamide, the intravenous dose is 250– 500 mg.
Side Effects
Carbonic anhydrase inhibitors generally produce only a mild hyperchloremic metabolic acidosis because of an apparently limited effect on the distal nephron. Large doses of acetazolamide have been reported to cause drowsiness, paresthesias, and con-fusion. Alkalinization of the urine can interfere with the excretion of amine drugs, such as quinidine. Acetazolamide is frequently used for prophylaxis against mountain sickness.
OTHER“DIURETICS”
These agents may increase GFR by elevating cardiac output or arterial blood pressure, thereby increasing RBF. Drugs in this category are not primarily classi-fied as diuretics because of their other major actions. They include methylxanthines (theophylline), cardiac glycosides (digitalis), fenoldopam (Corlopam), ino-tropes (dopamine, dobutamine), and intravenous crystalloid and colloid infusions. Methylxanthines also appear to decrease sodium reabsorption in both the proximal and distal renal tubules.
Related Topics