Chapter: Basic & Clinical Pharmacology : Antifungal Agents
Echinocandins
ECHINOCANDINS
Chemistry & Pharmacokinetics
Echinocandins are the
newest class of antifungal agents to be developed. They are large cyclic
peptides linked to a long-chain fatty acid. Caspofungin, micafungin, and anidulafungin
are the only licensed agents in this category of antifungals, although other
drugs are under active investigation. These agents are activeagainst Candida and Aspergillus, but not C
neoformans or the agents of zygomycosis and mucormycosis.
Echinocandins are
available only in intravenous formulations. Caspofungin is administered as a
single loading dose of 70 mg, followed by a daily dose of 50 mg. Caspofungin is
water soluble and highly protein-bound. The half-life is 9–11 hours, and the
metabolites are excreted by the kidneys and gastrointestinal tract. Dosage
adjustments are required only in the presence of severe hepatic insufficiency.
Micafungin displays similar properties with a half-life of 11–15 hours and is
used at a dose of 150 mg/d for treatment of esophageal candidiasis, 100 mg/d
for treatment of candidemia, and 50 mg/d for prophylaxis of fungal infections.
Anidulafungin has a half-life of 24–48 hours. For esophageal candidiasis, it is
administered intravenously at 100 mg on the first day and 50 mg/d thereafter
for 14 days. For candidemia, a loading dose of 200 mg is recommended with 100
mg/d thereafter for at least 14 days after the last positive blood culture.
Mechanism of Action
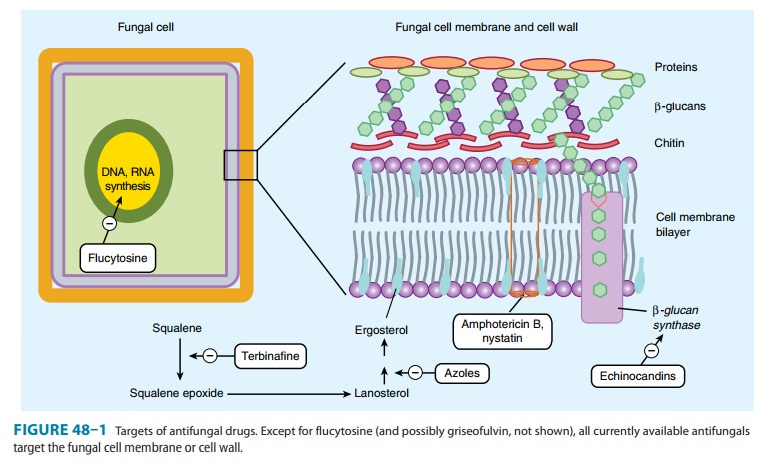
Echinocandins act at
the level of the fungal cell wall by inhibiting the synthesis of β(1–3)-glucan (Figure
48–1). This results in dis-ruption of the fungal cell wall and cell death.
Clinical Uses & Adverse Effects
Caspofungin is
currently licensed for disseminated and mucocuta-neous candidal infections, as
well as for empiric antifungal therapy during febrile neutropenia, and has
largely replaced amphotericin B for the latter indication. Of note, caspofungin
is licensed for use in invasive aspergillosis only as salvage therapy in
patients who have failed to respond to amphotericin B, and not as primary
therapy. Micafungin is licensed for mucocutaneous candidiasis, candidemia, and
prophylaxis of candidal infections in bone marrow transplant patients.
Anidulafungin is approved for use in esophageal candidi-asis and invasive
candidiasis, including candidemia.
Echinocandin agents
are extremely well tolerated, with minor gastrointestinal side effects and
flushing reported infrequently. Elevated liver enzymes have been noted in
several patients receiving caspofungin in combination with cyclosporine, and
this combina-tion should be avoided. Micafungin has been shown to increase
levels of nifedipine, cyclosporine, and sirolimus. Anidulafungin does not seem
to have significant drug interactions, but histamine release may occur during
intravenous infusion.
Related Topics