Chapter: Basic & Clinical Pharmacology : Antifungal Agents
Amphotericin B
SYSTEMIC ANTIFUNGAL DRUGS FOR SYSTEMIC INFECTIONS
AMPHOTERICIN B
Amphotericin
A and B are antifungal antibiotics produced by Streptomyces nodosus. Amphotericin A is not in clinical use.
Chemistry & Pharmacokinetics
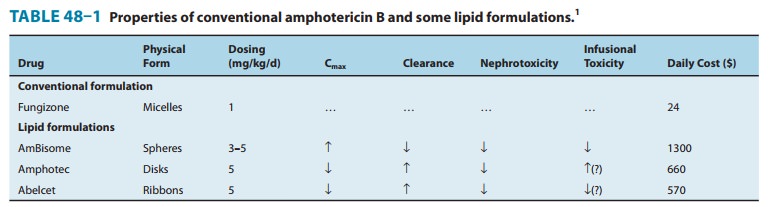
Amphotericin B is an amphoteric polyene macrolide (polyene = containing many double bonds; macrolide = containing a large lactone ring of 12 or more atoms). It is nearly insoluble in water and is therefore prepared as a colloidal suspension of amphotericin B and sodium desoxycholate for intravenous injection. Several formulations have been developed in which amphotericin B is packaged in a lipid-associated delivery system (Table 48–1 and Box: Liposomal Amphotericin B).
Amphotericin
B is poorly absorbed from the gastrointestinal tract. Oral amphotericin B is
thus effective only on fungi within the lumen of the tract and cannot be used
for treatment of systemic disease. The intravenous injection of 0.6 mg/kg/d of
amphotericin B results in average blood levels of 0.3–1 mcg/mL; the drug is
more than 90% bound by serum proteins. Although it is mostlymetabolized, some
amphotericin B is excreted slowly in the urine over a period of several days.
The serum half-life is approximately 15 days. Hepatic impairment, renal
impairment, and dialysis have little impact on drug concentrations, and
therefore no dose adjust-ment is required. The drug is widely distributed in
most tissues, but only 2–3% of the blood level is reached in cerebrospinal
fluid, thus occasionally necessitating intrathecal therapy for certain types of
fungal meningitis.
Mechanisms of Action & Resistance
Amphotericin
B is selective in its fungicidal effect because it exploits the difference in
lipid composition of fungal and mammalian cell membranes. Ergosterol, a cell membrane sterol, is found in the cell membrane
of fungi, whereas the predominant sterol of bacteria and human cells is cholesterol. Amphotericin B binds to
ergosterol and alters the permeability of the cell by forming amphotericin
B-associated pores in the cell membrane (Figure 48–1).
Liposomal Amphotericin B
Therapy with amphotericin B is often limited by toxicity,
espe-cially drug-induced renal impairment. This has led to the devel-opment of
lipid drug formulations on the assumption that lipid-packaged drug binds to the
mammalian membrane less readily, permitting the use of effective doses of the
drug with lower toxicity. Liposomal amphotericin preparations package the
active drug in lipid delivery vehicles, in contrast to the colloidal
suspensions, which were previously the only available forms. Amphotericin binds
to the lipids in these vehicles with an affinity between that for fungal
ergosterol and that for human choles-terol. The lipid vehicle then serves as an
amphotericin reservoir, reducing nonspecific binding to human cell membranes.
This preferential binding allows for a reduction of toxicity without
sacrificing efficacy and permits use of larger doses. Furthermore,some fungi
contain lipases that may liberate free amphotericin B directly at the site of
infection.
Three such formulations are now available and have differing pharmacologic properties as summarized in Table 48–1. Although clinical trials have demonstrated different renal and infusion-related toxicities for these preparations compared with regular amphotericin B, there are no trials comparing the different for-mulations with each other. Limited studies have suggested at best a moderate improvement in the clinical efficacy of the lipid formulations compared with conventional amphotericin B. Because the lipid preparations are much more expensive, their use is usually restricted to patients intolerant to, or not respond-ing to, conventional amphotericin treatment.
As
sug-gested by its chemistry, amphotericin B combines avidly with lipids (ergosterol) along the double bond-rich
side of its structure and associates with water molecules along the
hydroxyl-rich side. This amphipathic characteristic facilitates pore formation
by mul-tiple amphotericin molecules, with the lipophilic portions around the
outside of the pore and the hydrophilic regions lining the inside. The pore
allows the leakage of intracellular ions and mac-romolecules, eventually
leading to cell death. Some binding to human membrane sterols does occur,
probably accounting for the drug’s prominent toxicity.
Resistance to
amphotericin B occurs if ergosterol binding is impaired, either by decreasing
the membrane concentration of ergosterol or by modifying the sterol target
molecule to reduce its affinity for the drug.
Antifungal Activity & Clinical Uses
Amphotericin B remains
the antifungal agent with the broadest spectrum of action. It has activity
against the clinically significant yeasts, including Candida albicans and Cryptococcus
neoformans; the organisms causing endemic mycoses, including Histoplasmacapsulatum, Blastomyces dermatitidis, and Coccidioides immitis;and the pathogenic
molds, such as Aspergillus fumigatus
and the agents of mucormycosis. Some fungal organisms such as Candidalusitaniae and Pseudallescheria boydii display
intrinsic amphoteri-cin B resistance.
Owing
to its broad spectrum of activity and fungicidal action, amphotericin B remains
a useful agent for nearly all life-threatenin mycotic infections, although
newer, less toxic agents have largely replaced it for most conditions.
Amphotericin B is often used as the initial induction regimen to rapidly reduce
fungal burden and then replaced by one of the newer azole drugs (described
below) for chronic therapy or prevention of relapse. Such induction therapy is
especially important for immunosup-pressed patients and those with severe
fungal pneumonia, severe cryptococcal meningitis, or disseminated infections
with one of the endemic mycoses such as histoplasmosis or coccidioidomy-cosis.
Once a clinical response has been elicited, these patients then often continue
maintenance therapy with an azole; therapy may be lifelong in patients at high
risk for disease relapse. For treatment of systemic fungal disease,
amphotericin B is given by slow intravenous infusion at a dosage of 0.5–1
mg/kg/d. It is usually continued to a defined total dose (eg, 1–2 g), rather
than a defined time span, as used with other antimicrobial drugs.
Intrathecal therapy
for fungal meningitis is poorly tolerated and fraught with difficulties related
to maintaining cerebrospinal fluid access. Thus, intrathecal therapy with
amphotericin B is being increasingly supplanted by other therapies but remains
an option in cases of fungal central nervous system infections that have not
responded to other agents.
Local or topical
administration of amphotericin B has been used with success. Mycotic corneal
ulcers and keratitis can be cured with topical drops as well as by direct
subconjunctival injec-tion. Fungal arthritis has been treated with adjunctive
local injection directly into the joint. Candiduria responds to bladder
irrigation with amphotericin B, and this route has been shown to produce no
significant systemic toxicity.
Adverse Effects
The toxicity of
amphotericin B can be divided into two broad categories: immediate reactions,
related to the infusion of the drug, and those occurring more slowly.
A. Infusion-Related Toxicity
Infusion-related
reactions are nearly universal and consist of fever, chills, muscle spasms,
vomiting, headache, and hypoten-sion. They can be ameliorated by slowing the infusion
rate or decreasing the daily dose. Premedication with antipyretics,
anti-histamines, meperidine, or corticosteroids can be helpful. When starting
therapy, many clinicians administer a test dose of 1 mg intravenously to gauge
the severity of the reaction. This can serve as a guide to an initial dosing
regimen and premedication strategy.
B. Cumulative Toxicity
Renal
damage is the most significant toxic reaction. Renal impair-ment occurs in
nearly all patients treated with clinically significant doses of amphotericin.
The degree of azotemia is variable and often stabilizes during therapy, but it
can be serious enough to necessitate dialysis. A reversible component is
associated with decreased renal perfusion and represents a form of prerenal
renal failure. An irreversible component results from renal tubular injury and
subsequent dysfunction. The irreversible form of amphotericin nephrotoxicity
usually occurs in the setting of pro-longed administration (> 4 g
cumulative dose). Renal toxicity commonly manifests as renal tubular acidosis
and severe potas-sium and magnesium wasting. There is some evidence that the
prerenal component can be attenuated with sodium loading, and it is common
practice to administer normal saline infusions with the daily doses of
amphotericin B.
Abnormalities of liver
function tests are occasionally seen, as is a varying degree of anemia due to
reduced erythropoietin produc-tion by damaged renal tubular cells. After
intrathecal therapy with amphotericin, seizures and a chemical arachnoiditis
may develop, often with serious neurologic sequelae.
Related Topics