Chapter: Biology of Disease: Disorders of Acid Base Balance
Disorders of Acid Base Balance
DISORDERS OF ACID–BASE BALANCE
INTRODUCTION
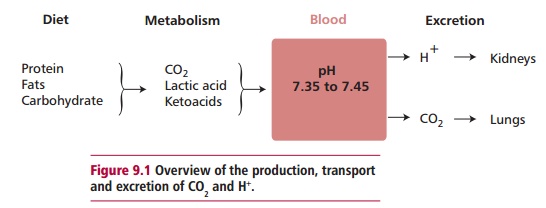
The concentration of hydrogen ions, H+, in the blood is
kept within a narrow reference range to give the blood a pH of approximately
7.4. The body possesses physiological and biochemical mechanisms that maintain
this pH by removing excess H+ and carbon dioxide produced during
metabolism (Figure9.1). These
activities are vital for normal bodily functions and are performedby the renal
and respiratory systems respectively. Failure to maintain the
acid–base balance at an appropriate value will give rise either
to an acidosis, with a blood pH below the reference range, or an alkalosis with
the pH above it. Different types of acidoses and alkaloses produce specific
characteristic clinical features. Once a specific acid–base disorder has been
identified, a clinical strategy must be adopted to manage the symptoms and to
treat the underlying cause(s).
Related Topics