Chapter: Biology of Disease: Disorders of Acid Base Balance
The Production and Transport of Carbon Dioxide
THE PRODUCTION AND TRANSPORT OF
CARBON DIOXIDE
Body tissues produce about 20 moles of CO2 per day
during oxidative metabolism. The CO2 diffuses from the cells into
the extracellular fluid (ECF), that is the blood and tissue fluid, and
eventually enters the plasma in quantities with the potential to form enough
carbonic acid to disturb its pH. However, in normal circumstances this does not
occur because the CO2 is transported to the lungs and excreted.
During transport, a substantial proportion of the CO2 enters the
erythrocytes by diffusion. Within the erythrocytes, a small proportion of the
CO2 remains dissolved or combines with proteins, mainly hemoglobin,
to form carbamino compounds:
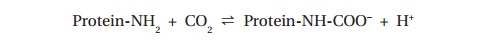
The major portion, however, combines with water to produce
carbonic acid in a reaction catalyzed by carbonic anhydrase (Figure 9.2):
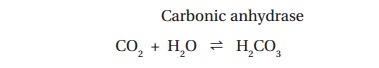
Carbonic acid dissociates to H+ and hydrogen
carbonate (HCO3ŌĆō, ŌĆśbicarbonateŌĆÖ)
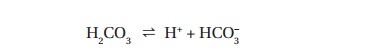
Figure 9.3 shows how H+are
removed from solution when they react withoxyhemoglobin (HbO8) and
promote the release of its oxygen to the tissues and forms protonated
hemoglobin (ŌĆśH+HbŌĆÖ). The HCO3ŌĆō formed diffuses
down its electrochemical gradient out of the erythrocytes to the plasma in
exchange for ClŌĆō, thus maintaining the electrochemical equilibrium
of the erythrocyte. The exchange of HCO3ŌĆō for ClŌĆō
is normally called the chloride shift.
Since both ions are charged, neither would pass freely across biological
membranes, however, an anion exchanger protein facilitates their transport.
This exchanger is a membrane protein that forms a pore through the membrane
allowing the cotransport of the ions across the membrane. Given that the ions
move in opposite directions, the anion exchanger or cotransporter is said to be
an antiporter. The concentration of HCO3ŌĆō in the plasma
is normally kept between 21ŌĆō28 mmol dmŌĆō3.
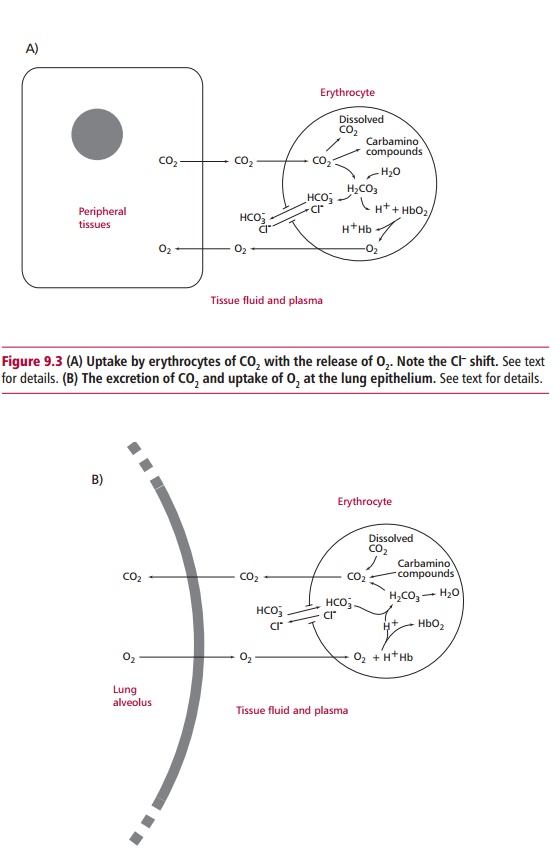
In the lungs, the partial pressure of oxygen is high while that
of carbon dioxide is low. Thus oxygen enters the erythrocytes forming
oxyhemoglobin, releasing the bound H+ and promoting the reverse of
the events that occur in other body tissues (Figure 9.3). Thus, H+ associates with HCO3ŌĆō
to produce carbonic acid which then breaks down to carbon dioxide and water.
The water enters the large body pool of water while the CO2 leaves
the erythrocytes and is excreted on exhalation.
These events provide an interesting confirmation that enzymes catalyze
reactions in either direction depending upon the position of equilibrium. Thus
carbonic anhydrase promotes the formation of carbonic acid in most body tissues
where the concentration of CO2 is relatively high. However, in the
lungs, where the concentration of CO2 is reduced, the enzyme
catalyzes the formation of CO2 and H2O from carbonic
acid.
Related Topics