Chapter: Biology of Disease: Disorders of Acid Base Balance
Buffering and the Excretion of H+
BUFFERING AND THE EXCRETION OF H+
About 60 mmol of H+ are produced each day from the
oxidation of sulfur-containing amino acid residues or from incomplete metabolic
activities, such as anerobic glucose metabolism or ketone body formation . If
all the H+ were released into the approximately 14 dm3 of
ECF, the concentration of H+ would be 4 mmol dm–3 or
about 100 000 times more acidic than normal. In reality, the concentration of H+
is kept within the narrow limits of 40 p5 nmol dm–3 to
maintain the appropriate body physiological pH of 7.4 ± 0.05. This pH is
necessary for normal physiological functions and is maintained by temporary buffering
systems that resist changes to the pH of the plasma until the excessive H+
are excreted by the kidneys .
When H+are released by cells, the ECF is buffered by
the hydrogen carbonate– carbonic acid buffer system:
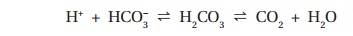
Other buffering systems, such as hemoglobin in the erythrocytes,
also make significant contributions as described. If the concentrations of H+
and HCO3– reach equilibrium, buffering would become
ineffective. However, in the case of the hydrogen carbonate–carbonic acid
system this is usually prevented from occurring by the breakdown of carbonic
acid to CO2 and water. The formation of carbonic acid from H+
and HCO3– is a rapid reaction. Its potentially slow
breakdown to CO2 and H2O is accelerated by carbonic
anhydrase in the erythrocytes and kidneys and the removal of carbon dioxide at
the lungs prevents the system from reaching equilibrium.
Buffering by the hydrogen carbonate–carbonic acid system removes
H+ from the ECF but at the expense of HCO3–.
The ECF contains relatively large amounts of HCO3–, for
example, its concentration is usually about 24 mmol dm–3. If, for
any reason, the amount of H+ produced increases, then the
concentration of HCO3– will decrease as the hydrogen
carbonate–carbonic acid buffering system operates. Any excess H+
must be excreted from the body by the kidneys
to keep the ECF and tissues at an appropriate pH. In addition,
the HCO3– used in buffering must be regenerated to return
its concentration in the plasma to normal values, otherwise the body will
become depleted of HCO3– and buffering capacity. Two
separate mechanisms operate in the kidneys to recover the HCO3–
initially removed from the blood by filtration at the glomerulus and to
regenerate that used in buffering. The first mechanism is the HCO3–
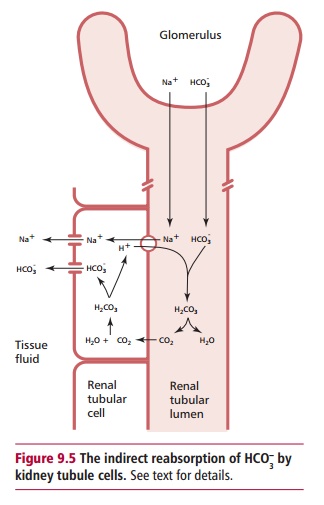
recovery system while the second regenerates the HCO3– (Figures9.5 and 9.6).
In health, virtually all of the HCO3– is
reabsorbed from the kidney tubule lumen. The operation of the system depends
upon the tubule cells being polarized, that is, their luminal and basal
surfaces differ in composition and permeability (Figure 9.5). In this manner, they resemble the enterocytes that
line the absorptive surface of the gastroinstinal tract . Direct reabsorption
of HCO3– from the renal tubular fluid cannot occur
because the luminal surfaces of renal tubular cells are impermeable to HCO3–.
However, the concentration of CO2 within the tubular cells is
maintained at a relatively high value and so carbonic anhydrase catalyzes the
formation of carbonic acid. The acid dissociates to HCO3–
and H+. The continuous formation of HCO3– and
H+ within the tubule cells is promoted by their removal. The HCO3–
is transported across the basal membrane of the cell into the interstitial
fluid and then into the capillaries. In contrast, H+ is exchanged
for Na+ across the luminal membrane and enters the lumen of the
kidney tubule. A membrane protein called the sodium bicarbonate cotransporter 1
(NBC 1) present in the luminal cell membrane facilitates the exchange of ions.
Within the lumen, the H+combine with HCO3– to
form carbonic acid. The acid breaks down spontaneously to CO2 and H2O
in the proximal tubule, but carbonic anhydrase activity on the luminal surfaces
of the cells speeds up the reaction in the distal tubule. The CO2
can enter the tubule cell across its luminal membrane and so the HCO3–
is recovered, indirectly, as CO2. Approximately 80% of the filtered
HCO3– in the proximal tubule is recovered by this
mechanism. However, there
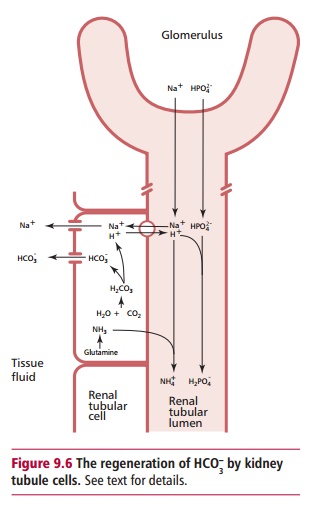
has not been a net loss of H+ and the HCO3–
used in buffering has not been regenerated. The HCO3– is
regenerated when carbonic acid is formed in the luminal cell as described above
(Figure 9.6). Again, H+are
exchanged for Na+ and enter the lumen. Here the H+ react
with phosphate (HPO42–) and ammonia (NH3) to
give H2PO4– and NH4+
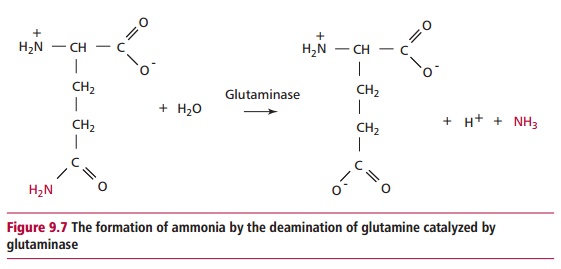
respectively. Ammonia is a significant urinary buffer produced by the
deamination of glutamine in the renal tubular cells in a reaction catalyzed by
glutaminase (Figure 9.7). The ammonia
formed can readily diffuse across cell membranes but NH4+
cannot enter the cells by passive reabsorption. Thus the NH4+,
and the H2PO4–,
are excreted in the urine. For every H+ excreted as NH 4+
and H2PO4–,
a single HCO3– is formed in the tubule cell and secreted
across the basal surface to the interstitial fluid and then into the blood.
Hence the HCO3– concentration of the ECF is regenerated.
The synthesis of glutaminase is induced in states of chronic
acidosis allowing an increase in the production of ammonia and an
increasedexcretion of H+ as NH4+.
Related Topics