Chapter: Environmental Engineering : Water Treatment
Demineralization : Principles,Equipment, Operation, Advantages And Limitations
DEMINERALIZATION
Softening alone is insufficient for most high-pressure boiler
feedwaters and for many process streams, especially those used in the
manufacture of electronics equipment. In addition to the removal of hardness,
these processes require removal of all dissolved solids, such as sodium,
silica, alkalinity, and the mineral anions (Cl¯ , SO4²¯ , NO3¯
).
Demineralization of water is the
removal of essentially all inorganic salts by ion exchange. In this process,
strong acid cation resin in the hydrogen form converts dissolved salts into
their corresponding acids, and strong base anion resin in the hydroxide form
removes these acids. Demineralization produces water similar in quality to
distillation at a lower cost for most fresh
Principles
of Demineralization
A demineralizer system consists
of one or more ion exchange resin columns, which include a strong acid cation
unit and a strong base anion unit. The cation resin exchanges hydrogen for the
raw water cations as shown by the following reactions:
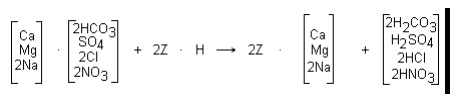
A measure of the total concentration of the strong acids in
the cation effluent is the free mineral acidity (FMA). In a typical service run,
the FMA content is stable most of the time, as shown in Figure 8-8. If cation
exchange were 100% efficient, the FMA from the exchanger would be equal to the
theoretical mineral acidity (TMA) of the water. The FMA is usually slightly
lower than the TMA because a small amount of sodium leaks through the cation
exchanger. The amount of sodium leakage depends on the regenerant level, the
flow rate, and the proportion of sodium to the other cations in the raw water.
In general, sodium leakage increases as the ratio of sodium to total cations
increases.
As a cation exchange unit nears exhaustion, FMA in the
effluent drops sharply, indicating that the exchanger should be removed from
service. At this time the resin should be regenerated with an acid solution, which
returns the exchange sites to the hydrogen form. Sulfuric acid is normally used
due to its affordable cost and its availability. However, improper use of
sulfuric acid can cause irreversible fouling of the resin with calcium sulfate.
To prevent this occurrence, the sulfuric acid is usually
applied at a high flow rate (1 gpm per square foot of resin) and an initial
concentration of 2% or less. The acid concentration is gradually increased to
6-8% to complete regeneration.
Some installations use hydrochloric acid for regeneration.
This necessitates the use of special materials of construction in the
regenerant system. As with a sodium zeolite unit, an excess of regenerant
(sulfuric or hydrochloric acid) is required up to three times the theoretical dose.
To complete the demineralization
process, water from the cation unit is passed through a strong base anion
exchange resin in the hydroxide form. The resin exchanges hydrogen ions for
both highly ionized mineral ions and the more weakly ionized carbonic and
silicic acids, as shown below:
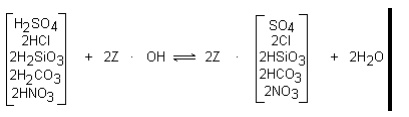
The above reactions indicate that demineralization completely
removes the cations and anions from the water. In reality, because ion exchange
reactions are equilibrium reactions, some leakage occurs. Most leakage from
cation units is sodium. This sodium leakage is converted to sodium hydroxide in
the anion units. There-fore, the effluent pH of a two bed cation-anion demineralizer system is slightly alkaline.
The caustic produced in the anions causes a small amount of silica leakage. The
extent of leakage from the anions depends on the chemistry of the water being
processed and the regenerant dosage being used.
Demineralization using strong anion resins removes silica as
well as other dissolved solids. Effluent silica and conductivity are important
parameters to monitor during a demineralizer service run. Both silica and
conductivity are low at the end of the fast rinse, as shown in Figure 8-9.
When silica breakthrough occurs at the end of a service run,
the treated water silica level increases sharply. Often, the conductivity of
the water decreases momentarily, then rises rapidly. This temporary drop in
conductivity is easily explained. During the normal service run, most of the
effluent conductivity is attributed to the small level of sodium hydroxide
produced in the anion exchanger. When silica breakthrough occurs, the hydroxide
is no longer available, and the sodium from the cation exchanger is converted
to sodium silicate, which is much less conductive than sodium hydroxide. As
anion resin exhaustion progresses, the more conductive mineral ions break
through, causing a subsequent increase in conductivity.
When the end of a demineralizer run is detected, the unit must
be removed from service immediately. If the demineralizer is allowed to remain
in service past the breakpoint, the level of silica in the treated water can
rise above that of the influent water, due to the concentrating of silica that
takes place in the anion resin during the service run.
Strong base anion exchangers are regenerated with a 4% sodium
hydroxide solution. As with cation regeneration, the relatively high
concentration of hydroxide drives the regeneration reaction. To improve the
removal of silica from the resin bed, the regenerant caustic is usually heated
to 120 o F or to the temperature specified by the resin manufacturer. Silica
removal is also enhanced by a resin bed preheat step before the introduction of
warm caustic.
Equipment
and Operation
The equipment used for cation-anion demineralization is
similar to that used in zeolite softening. The primary difference is that the
vessels, valves, and piping must be made of (or lined with) corrosion-resistant
materials. Rubber and polyvinyl chloride (PVC) are commonly used for ion
exchange vessel linings. The controls and regenerant systems for demineralizers
are more complex, to allow for such enhancements as stepwise acid and warm
caustic regenerations.
Demineralizers are similar in operation to zeolite softeners.
The service flow rate guidelines for a demineralizer range from 6 to 10 gpm per
square foot of resin. Flow rates of over 10 gpm per square foot of resin cause
increased sodium and silica leakage with certain waters. Anion resin is much
lighter than cation resin. Therefore, the backwash flow rates for anion
exchange resins are much lower than those for cation resins, and anion resin
expansion is affected by the temperature of the water more than cation resin
expansion. The water used for each step of anion resin regeneration should be
free from hardness, to prevent precipitation of hardness salts in the alkaline
anion resin bed.
Continuous
conductivity instruments and silica analyzers are commonly used to monitor
anion effluent water quality and detect the need for regeneration. In some
instances, conductivity probes are placed in the resin bed above the underdrain
collectors to detect resin exhaustion before silica breakthrough into the
treated water occurs.
Advantages
and Limitations
Demineralizers can produce high-purity water for nearly every
use. Demineralized water is widely used for high pressure boiler feedwater and
for many process waters. The quality of water produced is comparable to
distilled water, usually at a fraction of the cost. Demineralizers come in a
wide variety of sizes. Systems range from laboratory columns that produce only
a few gallons per hour to systems that produce thousands of gallons per minute.
Like other ion exchange systems,
demineralizers require filtered water in order to function efficiently. Resin
foulants and degrading agents, such as iron and chlorine, should be avoided or
removed prior to demineralization. Anion resins are very susceptible to fouling
and attack from the organic materials present in many surface water supplies.
Some forms of silica, known as colloidal, or non-reactive, are not removed by a
demineralizer. Hot, alkaline boiler water dissolves the colloidal material,
forming simple silicates that are similar to those that enter the boiler in a
soluble form. As such, they can form deposits on tube surfaces and volatilize
into the steam.
Related Topics