Chapter: Environmental Engineering : Water Treatment
Dealkalization
DEALKALIZATION
Often, boiler or process operating conditions
require the removal of hardness and the reduction of alkalinity but not the
removal of the other solids. Zeolite softening does not reduce alkalinity, and
demineralization is too costly. For these situations, a dealkalization process
is used. Sodium zeolite/hydrogen zeolite (split stream) dealkalization,
chloride-anion dealkalization, and weak acid cation dealkalization are the most
frequently used processes.
Sodium
Zeolite/Hydrogen Zeolite (Split Stream) Dealkalization
In a split stream dealkalizer, a portion of the raw
water flows through a sodium zeolite softener. The remainder flows through a
hydrogen-form strong acid cation unit (hydrogen zeolite). The effluent from the
sodium zeolite is combined with the hydrogen zeolite effluent. The effluent
from the hydrogen zeolite unit contains carbonic acid, produced from the raw
water alkalinity, and free mineral acids. When the two streams are combined,
free mineral acidity in the hydrogen zeolite effluent converts sodium carbonate
and bicarbonate alkalinity in the sodium zeolite effluent to carbonic acid as
shown below:
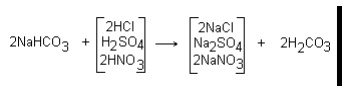
Carbonic acid is unstable in water. It forms carbon
dioxide gas and water. The blended effluents are sent to a decarbonator or
degasser, where the carbon dioxide is stripped from the water by a
countercurrent stream of air.
The desired level of blended water alkalinity can be
maintained through control of the percentage of sodium zeolite and hydrogen
zeolite water in the mixture. A higher percentage of sodium zeolite water
results in higher alkalinity, and an increased percentage of hydrogen zeolite
water reduces alkalinity.
In addition to reducing alkalinity, a split stream
dealkalizer reduces the total dissolved solids of the water. This is important
in high alkalinity waters, because the conductivity of these waters affects the
process and can limit boiler cycles of concentration.
Sodium
Zeolite/Chloride Anion Dealkalization
Strong base anion resin in the chloride form can be
used to reduce the alkalinity of a water. Water flows through a zeolite
softener and then an anion unit, which replaces the carbonate, bicarbonate,
sulfate, and nitrate ions with chloride ions as shown in these reactions:
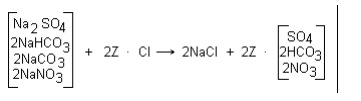
The chloride anion dealkalizer reduces alkalinity by
approximately 90% but does not reduce total solids. When the resin nears
exhaustion, treated water alkalinity increases rapidly, signaling the need for
regeneration.
The zeolite softener is regenerated as previously
described. In addition, the anion resin is also regenerated with a sodium
chloride brine that returns the resin to the chloride form. Frequently, a small
amount of caustic soda is added to the regenerant brine to enhance alkalinity
removal.
Weak
Acid Cation Dealkalization
Another method of dealkalization uses weak acid
cation resins. Weak acid resins are similar in operation to strong acid cation
resins, but only exchange for cations that are associated with alkalinity, as
shown by these reactions:
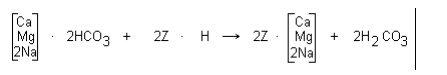
where Z represents the resin. The carbonic acid (H2CO3)
formed is removed by a decarbonator or degasser as in a split stream system.
The ideal influent for a weak acid cation system has
a hardness level equal to the alkalinity (both expressed in ppm as CaCO3).
In waters that are higher in alkalinity than hardness, the alkalinity is not
removed to its lowest level. In waters containing more hardness than
alkalinity, some hardness remains after treatment. Usually, these waters must
be polished by a sodium zeolite softener to remove hardness. During the initial
portion of a weak acid cation service run (the first 40-60%) some cations
associated with mineral anions exchange, producing small amounts of mineral
acids in the effluent. As the service cycle progresses, alkalinity appears in
the effluent. When the alkalinity in the effluent exceeds 10% of the influent
alkalinity, the unit is removed from service and regenerated with a 0.5%
sulfuric acid solution. The concentration of regenerant acid should be kept
below 0.5-0.7%, to prevent calcium sulfate precipitation in the resin. Weak
acid cation resin exchange is very efficient. Therefore, the amount of acid required
is virtually equal (chemically) to the amount of cations removed during the
service cycle.
If
the materials of construction for the down-stream equipment or overall process
cannot tolerate the mineral acidity present during the initial portions of the
service cycle, a brine solution is passed through the regenerated weak acid
resin prior to the final rinse. This solution removes the mineral acidity
without a significant impact on the quality or length of the subsequent run.
Equipment
used for a weak acid cation dealkalizer is similar to that used for a strong
acid cation exchanger, with the exception of the resin. One variation of the
standard design uses a layer of weak acid resin on top of strong acid cation
resin. Because it is lighter, the weak acid resin remains on top. The layered
resin system is regenerated with sulfuric acid and then with sodium chloride
brine. The brine solution converts the strong acid resin to the sodium form.
This resin then acts as a polishing softener.
Direct Acid Injection
In
the process of direct acid injection and decarbonation, acid is used to convert
alkalinity to carbonic acid. The carbonic acid dissociates to form carbon
dioxide and water and the carbon dioxide is removed in a decarbonator. The use
of an acid injection system should be approached with caution, because an acid
overfeed or a breakdown in the pH control system can produce acidic feedwater,
which corrodes the iron surfaces of feedwater systems and boilers. Proper pH
monitoring and controlled caustic feed after decarbonation are required.
Advantages and
Limitations of Dealkalization Systems
Ion
exchange dealkalization systems produce hardness-free, low-alkalinity water at
a reasonable cost, and with a high degree of reliability. They are well suited
for processing feedwater for medium-pressure boilers, and for process water for
the beverage industry. Split stream and weak acid cation systems also reduce
the total dissolved solids. In addition to these advantages, the following
disadvantages must be considered:
·
dealkalizers do not remove all of the
alkalinity and do not affect the silica content of a water
·
dealkalizers require the same influent
purity as other ion exchange processes; filtered water that is low in potential
foulants must be used
·
the water produced by a dealkalization
system using a forced draft decarbonator becomes saturated with oxygen, so it
is potentially corrosive
Related Topics