Chapter: Biochemistry: Protein Purification and Characterization Techniques
Column Chromatography
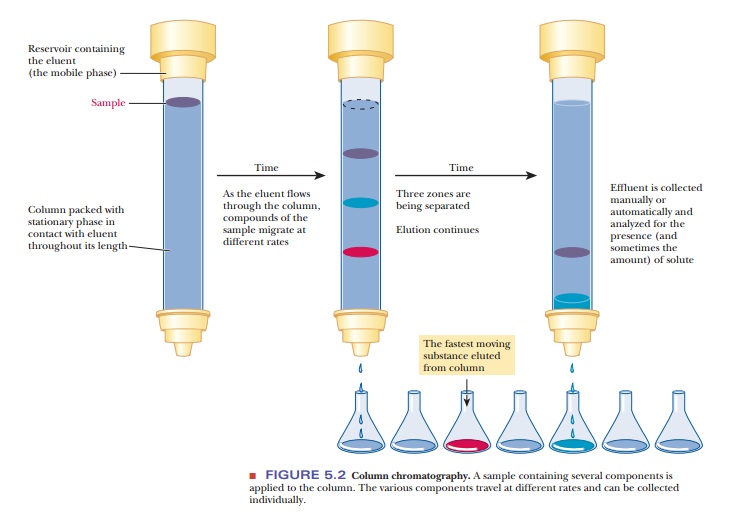
Column Chromatography
The word chromatography comes from the Greek chroma, “color,” and graphein, “to write”; the technique was first used around the beginning of the 20th century to separate plant pigments with easily visible colors. It has long since been possible to separate colorless compounds, as long as methods exist for detecting them. Chromatography is based on the fact that different compounds can distribute themselves to varying extents between different phases, or separable portions of matter. One phase is the stationary phase, and the other is the mobile phase.
The
mobile phase flows over the stationary material and carries the sample to be
separated along with it. The components of the sample interact with the
stationary phase to different extents. Some components interact relatively
strongly with the stationary phase and are therefore carried along more slowly
by the mobile phase than are those that interact less strongly. The differing
mobilities of the components are the basis of the separation.
Many
chromatographic techniques used for research on proteins are forms of column chromatography, in which the
material that makes up the stationary phase is packed in a column. The sample
is a small volume of concentrated solution that is applied to the top of the
column; the mobile phase, called the eluent,
is passed through the column. The sample is diluted by the eluent, andthe
separation process also increases the volume occupied by the sample. In a
successful experiment, the entire sample eventually comes off the column.
Figure 5.2 diagrams an example of column chromatography.
What are the different types of chromatography?
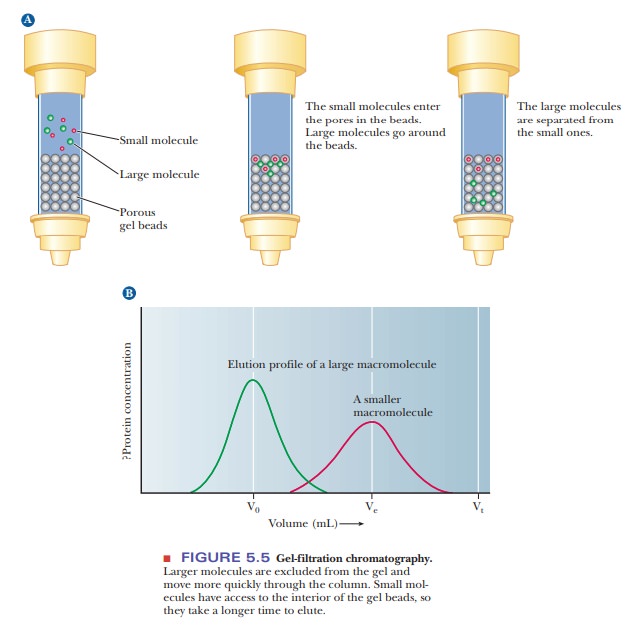
Size-exclusion chromatography, also called gel-filtration chromatography, separ-ates molecules on the basis of size, making it a useful way to sort proteins of varied molecular weights. It is a form of column chromatography in which the stationary phase consists of cross-linked gel particles. The gel particles are usually in bead form and consist of one of two kinds of polymers.
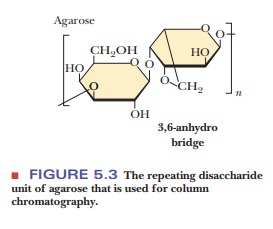
![]()
![]() The first is a carbohydrate
polymer, such as dextran or agarose; these two polymers are often
referred to by the trade names Sephadex and Sepharose, respectively (Figure
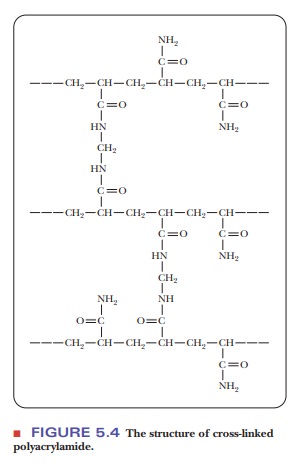
5.3). The second is based on polyacrylamide
(Figure 5.4), which is sold under the trade name Bio-Gel. The cross-linked
structure of these polymers produces pores in the material. The extent of
cross-linking can be controlled to select a desired pore size. When a sample is
applied to the column, smaller molecules, which are able to enter the pores,
tend to be delayed in their progress down the column, unlike the larger molecules.
As a result, the larger molecules are eluted first, followed later by the
smaller ones, after escaping from the pores. Molecular-sieve chromatography is
represented schematically in Figure 5.5. The advantages of this type of
chromatography are (1) its convenience as a way to separate molecules on the
basis of size and (2) the fact that it can be used to estimate molecular weight
by comparing the sample with a set of standards. Each type of gel used has a
specific range of sizes that separate linearly with the log of the molecular
weight. Each gel also has an exclusion limit, a size of protein that is too
large to fit inside the pores. All proteins that size or larger elute first and
simultaneously.
The first is a carbohydrate
polymer, such as dextran or agarose; these two polymers are often
referred to by the trade names Sephadex and Sepharose, respectively (Figure
5.3). The second is based on polyacrylamide
(Figure 5.4), which is sold under the trade name Bio-Gel. The cross-linked
structure of these polymers produces pores in the material. The extent of
cross-linking can be controlled to select a desired pore size. When a sample is
applied to the column, smaller molecules, which are able to enter the pores,
tend to be delayed in their progress down the column, unlike the larger molecules.
As a result, the larger molecules are eluted first, followed later by the
smaller ones, after escaping from the pores. Molecular-sieve chromatography is
represented schematically in Figure 5.5. The advantages of this type of
chromatography are (1) its convenience as a way to separate molecules on the
basis of size and (2) the fact that it can be used to estimate molecular weight
by comparing the sample with a set of standards. Each type of gel used has a
specific range of sizes that separate linearly with the log of the molecular
weight. Each gel also has an exclusion limit, a size of protein that is too
large to fit inside the pores. All proteins that size or larger elute first and
simultaneously.
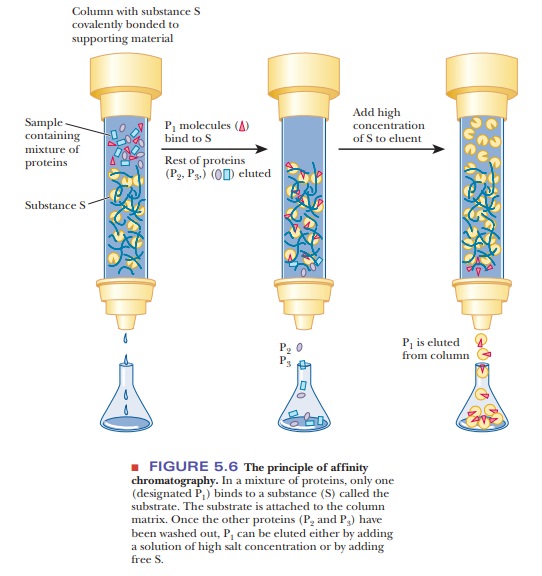
Affinity chromatography uses the
specific binding properties of many pro-teins. It is another form of column
chromatography with a polymeric material used as the stationary phase. The
distinguishing feature of affinity chroma-tography is that the polymer is
covalently linked to some compound, called a ligand, that binds specifically to the desired protein (Figure
5.6). The other proteins in the sample do not bind to the column and can easily
be eluted with buffer, while the bound protein remains on the column. The bound
protein can then be eluted from the column by adding high concentrations of the
ligand in soluble form, thus competing for the binding of the protein with the
stationary phase. The protein binds to the ligand in the mobile phase and is
recovered from the column. This protein–ligand interaction can also be
disrupted with a change in pH or ionic strength. Affinity chromatography is a
convenient separation method and has the advantage of producing very pure
proteins.
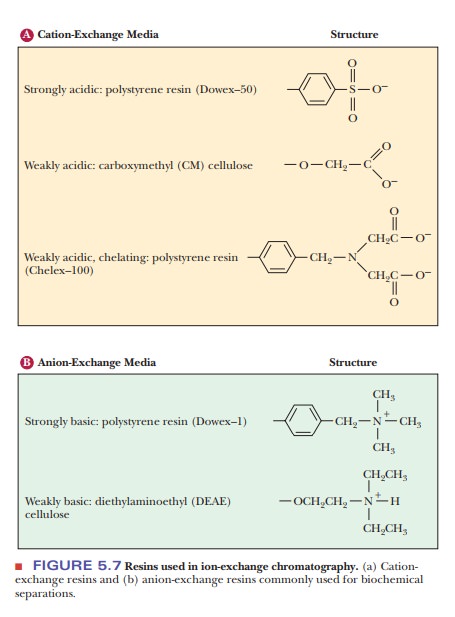
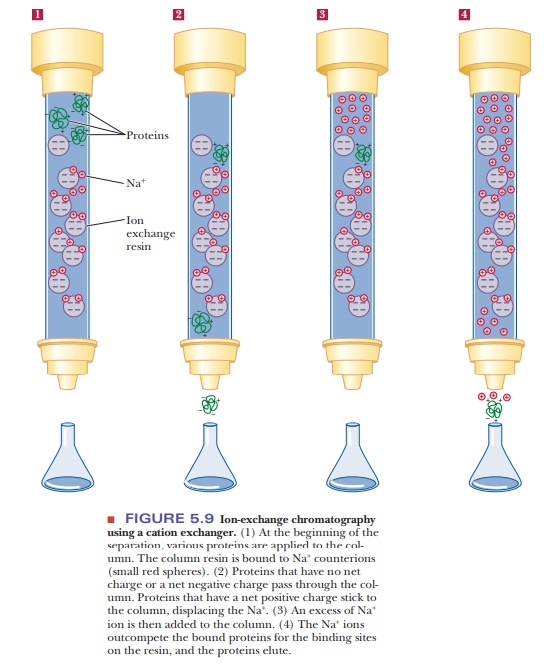
Ion-exchange chromatography is logistically similar to affinity
chroma-tography. Both use a column resin that binds the protein of interest.
With ion-exchange chromatography, however, the interaction is less specific and
is based on net charge. An ion-exchange resin has a ligand with a positive
charge or a negative charge. A negatively charged resin is a cation exchanger, and a positively
charged one is an anion exchanger.
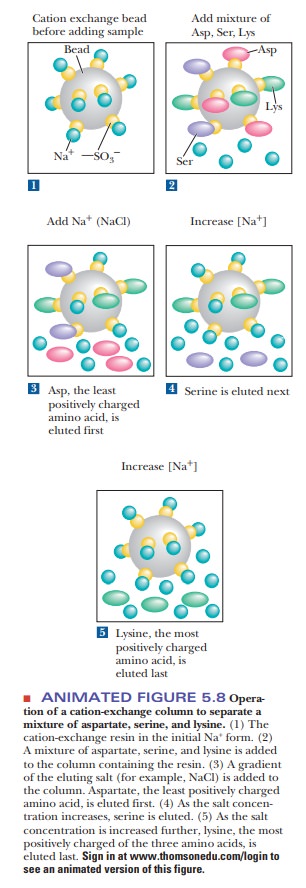
Figure 5.7 shows some typical ion-exchange ligands. Figure 5.8 illustrates
their principle of operation with three amino acids of different charge. Figure
5.9 shows how cation exchange chromatography would separate proteins. The
column is initially equilibrated with a buffer of suitable pH and ionic
strength. The exchange resin is bound to counterions. A cation-exchange resin
is usually bound to Na+ or K+ ions, and an anion
exchanger is usually bound to Cl– ions. A mixture of proteins is
loaded on the column and allowed to flow through it. Proteins that have a net
charge opposite to that of the exchanger stick to the column, exchanging places with the bound
counterions. Proteins that have no net charge or have the same charge as the
exchanger elute. After all the nonbinding proteins are eluted, the eluent is
changed either to a buffer that has a pH that removes the charge on the bound
proteins or to one with a higher salt concentration. The latter outcompetes the
bound proteins for the limited binding space on the column. The once-bound
molecules then elute, having been separated from many of the contaminating
ones.
Summary
Column chromatography refers to several common
techniques for purifi-cation of proteins.
In gel-filtration chromatography, proteins are separated by size.
In ion-exchange chromatography, molecules with
a specific charge are selectively bound to a column, separated from proteins
that don’t bind, and then eluted.
In
affinity chromatography, molecules are bound to the column via specific
interactions for a bound ligand. Once nonbinding proteins are removed, the
protein of interest can be eluted.
Related Topics