Chapter: Biochemistry: Protein Purification and Characterization Techniques
Cleavage of the Protein into Peptides
Cleavage of the Protein into
Peptides
Proteins can be cleaved at specific sites by enzymes or by chemical reagents. The enzyme trypsin cleaves peptide bonds preferentially at amino acids that have positively charged R groups, such as lysine and arginine.
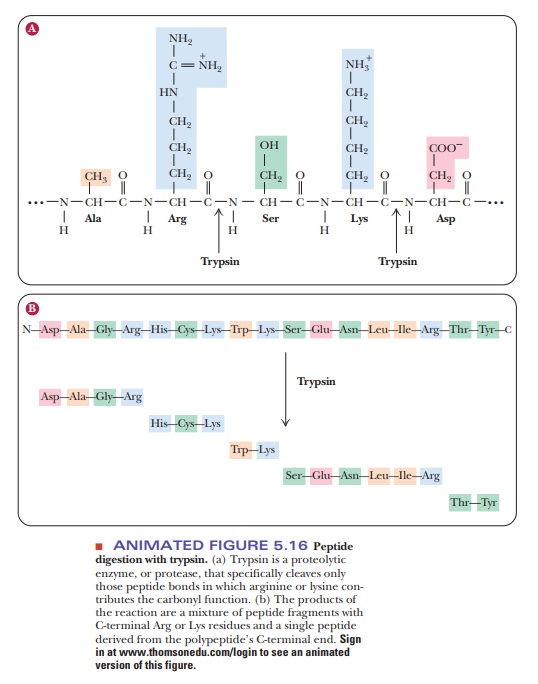
The cleavage takes place in
such a way that the amino acid with the charged side chain ends up at the
C-terminal end of one of the peptides produced by the reaction (Figure 5.16).
The C-terminal amino acid of the original protein can be any one of the 20
amino acids and is not necessarily one at which cleavage takes place. A peptide
can be automatically identified as the C-terminal end of the original chain if
its C-terminal amino acid is not a site of cleavage.
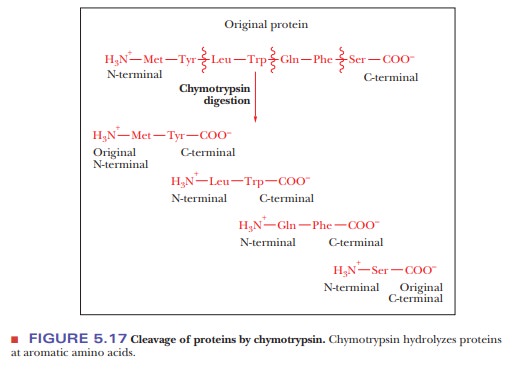
Another
enzyme, chymotrypsin, cleaves
peptide bonds preferentially at the aromatic amino acids: tyrosine, tryptophan,
and phenylalanine. The aromatic amino acid ends up at the C-terminal ends of
the peptides produced by the reaction (Figure 5.17).
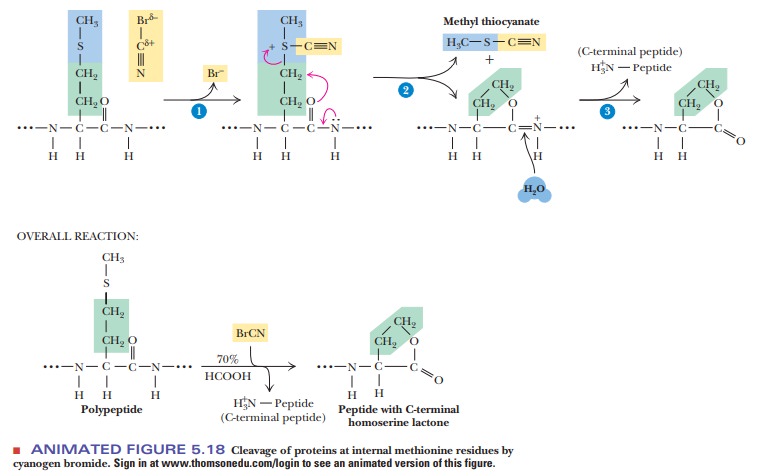
In the
case of the chemical reagent cyanogen
bromide (CNBr), the sites of cleavage are at internal methionine residues.
The sulfur of the methionine reacts with the carbon of the cyanogen bromide to
produce a homoserine lac-tone at the C-terminal end of the fragment (Figure
5.18).
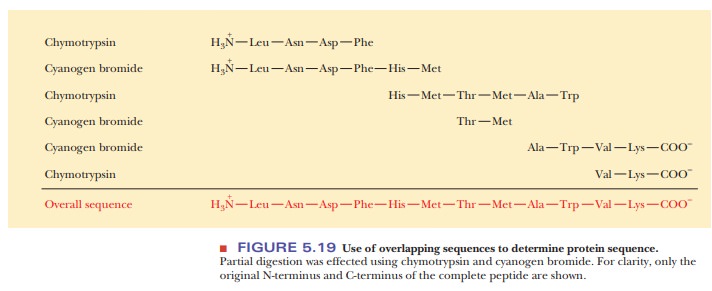
The
cleavage of a protein by any of these reagents produces a mixture of peptides,
which are then separated by high-performance liquid chromatogra-phy. The use of
several such reagents on different samples of a protein to be sequenced
produces different mixtures. The sequences of a set of peptides produced by one
reagent overlap the sequences produced by another reagent (Figure 5.19). As a
result, the peptides can be arranged in the proper order after their own
sequences have been determined.
Related Topics