Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Follicle-Stimulating Hormone
Clinical Aspects of Follicle Stimulating Hormone(FSH)
CLINICAL ASPECTS
Both recombinant FSH products on the market have been approved for two female indications. The first indication is anovulation (including polycystic ovar-ian disease) in women who are unresponsive to clomiphene citrate. The second indication is con-trolled ovarian hyperstimulation to induce the devel-opment of multiple follicles in medically assisted reproduction programs, such as IVF and embryo transfer. In addition, recombinant FSH may be used in men with congenital or required hypogonadotropic hypogonadism to stimulate spermatogenesis.
In anovulatory infertility in females, FSH treat-ment aims for the development of a single follicle, whereas IVF FSH treatment is aimed at multifollicular development. For the treatment of anovulatory patients it is recommended to start Puregon.
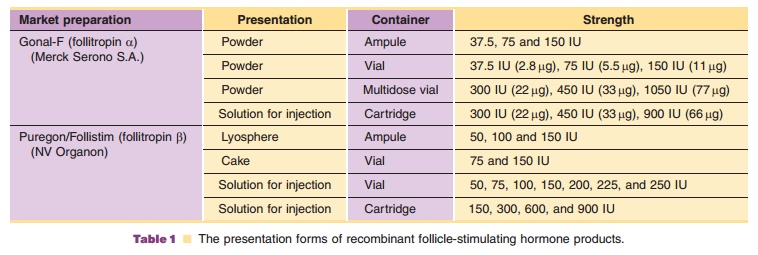
Follistim treatment with 50 IU per day for 7 to 14 days and gradually increase dosing with steps of 50 IU if no sufficient response is seen. This gradual dose-increasing schedule is followed in order to prevent multifollicular development and the induc-tion of ovarian hyperstimulation syndrome (a serious condition of unwanted hyperstimulation).
In the most commonly applied treatment regi-mens in IVF, endogenous gonadotropin levels are suppressed by a GnRH agonist or by the more recently approved GnRH antagonists (Cetrotide and Orgalutran /Antagon ). It is recommended to start Puregon treatment with 100 to 200 IU of recombinant FSH followed by maintenance doses of 50 to 350 IU. The availability of a surplus of collected oocytes allows the replacement of two to three embryos. Similar treatment regimens are recom-mended for Gonal-F .
Serum levels of FSH do not correlate with pharmacological responses, such as the production of estradiol, inhibin response or follicular develop-ment. Moreover, there is a large variability found in the individual pharmacological response to a fixed dose of recombinant FSH. This variability emphasizes the importance of a patient individualized dosing and treatment regimen when using recombinant FSH.
Follitropin b has an elimination half-life of approximately 40 hours. Steady-state levels of follitro-pin b are therefore reached after four daily doses. At that time, the concentrations of circulating immunor-eactive FSH are about a factor of 1.5–2.5 higher than after a single dose. This increase is relevant in attaining therapeutically effective plasma concentrations of FSH. Follitropin b can be administered via the intramuscular, as well as via the subcutaneous route, because the absence of impurities results in an improved local tolerance. Bioavailability via both routes is approxi-mately 77%. Injections of the highly pure follitropin preparations do not require medical personnel, but can be given by the patient herself or her partner. In a large number of patients treated with follitropin b, no formation of antibodies against recombinant FSH or CHO-cell derived proteins was observed.
Differences with Urinary FSH Preparations
In IVF, follitropin b treatment was found to be more effective and efficient than treatment with urinary FSH, since more follicles, oocytes, embryos and pregnancies are obtained with a lower total dose of recombinant FSH in a shorter treatment period. In a recent meta analysis the occurrence of higher pregnancy rates with recombi-nant FSH versus urinary FSH was confirmed (Daya, 2002). Treatment of patients with anovulatory infertility with either follitropin or urinary FSH was equally effective, but follitropin treatment was more efficient(a lower total dose was needed in a shorter duration of treatment).
Related Topics