Chapter: Basic Electrical and electronics : Semiconductor Devices And Applications
Classification of Semiconductor
Classification of Semiconductor
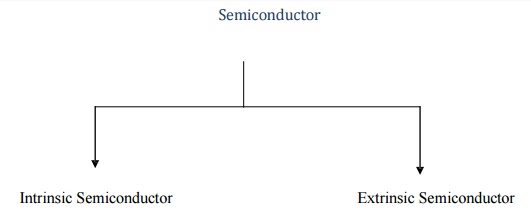
Intrinsic Semiconductor
An intrinsic semiconductor also called an undoped semiconductor or i- type semiconductor.
It is a pure semiconductor without any significant dopant species present.
The number of charge carriers determined by the properties of the m aterial itself instead of the amount of impurities.
In intrinsic semiconductors the number of excited electrons and the number of holes are equal: n = p.
Conductivity of Intrinsic semiconductor
The electrical conductivity of
intrinsic semiconductors can be due to crystal defects or to thermal
excitation.
Both electrons and holes
contribute to current flow in an intrinsic semiconductor.
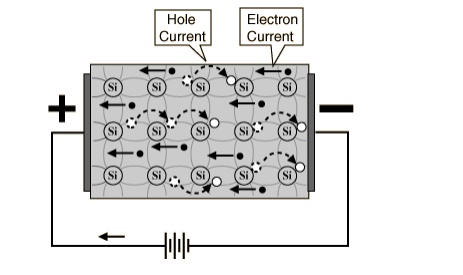
The
current which will flow in an intrinsic semiconductor consists of both electron
and hole current.
That
is, the electrons which have been freed from their lattice positions into the
conduction band can move through the material.
In
addition, other electrons can hop between lattice positions to fill the
vacancies left by the freed electrons.
This
additional mechanism is called hole conduction because it is as if the holes
are migrating across the material in the direction opposite to the free
electron movement.
The current flow in an intrinsic
semiconductor is influenced by the density of energy states which in turn
influences the electron density in the conduction band.
This
current is highly temperature dependent.
Thermal
excitation:
In
an intrinsic semiconductor like silicon at temperatures above absolute zero,
there will be some electrons which are excited across the band gap into the
conduction band and which can produce current.
When
the electron in pure silicon crosses the gap, it leaves behind an electron
vacancy or "hole" in the regular silicon lattice.
Under
the influence of an external voltage, both the electron and the hole can move
across the material.
In
n-type semiconductor:
The dopant contributes extra
electrons, dramatically increasing the conductivity
In p-type semiconductor:
The dopant produces extra vacancies
or holes, which likewise increase the conductivity.
Extrinsic
Semiconductor
The electrical conductivity of a
pure semiconductor is very small.
To increase the conductivity,
impurities are added.
The impurity added semiconductor
is called extrinsic semiconductor.
The process of adding impurity is
called doping.
The added impurity is called
dopant.
Usually one or two atoms of
impurity is added per 106 atoms of a semiconductor.
There are two types (i) p-type and
(ii) n-type semiconductors.
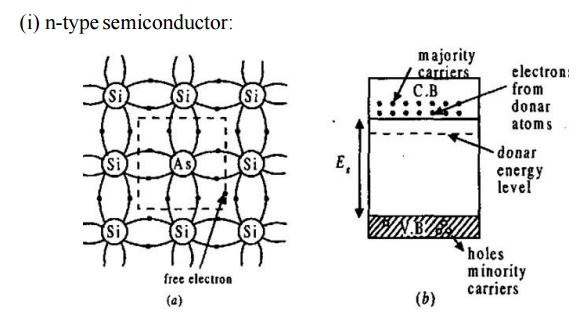
When an impurity, from V group elements like arsenic (As), antimony
having 5 valence electrons is added to Ge (or Si), the impurity atom donates
one electron to Ge (or Si).
The
4 electrons of the impurity atom is engaged in covalent bonding with Si atom.
The
fifth electron is free. This increases the conductivity.
The
impurities are called donors.
The
impurity added semiconductor is called n-type semiconductor, because their
increased conductivity is due to the presence of the negatively charged
electrons, which are called the majority carriers.
The
energy band of the electrons donated by the impurity atoms is just below the
conduction band.
The
electrons absorb thermal energy and occupy the conduction band.
Due to the breaking of covalent
bond, there will be a few holes in the valence band at this temperature.
These
holes in n-type are called minority carriers.
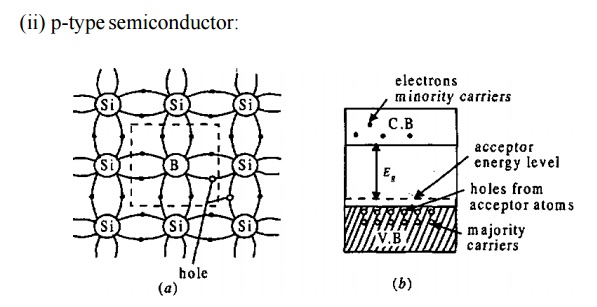
If a III group element, like
indium (In), boron (B), aluminium (AI) etc., having three valence electrons, is
added to a semiconductor say Si, the three electrons form covalent bond.
There is a deficiency of one electron to complete the 4th covalent bond and is called a hole.The presence of the hole increases the conductivity because these holes move to the nearby atom, at the same time the electrons move in the opposite direction.
The
impurities added semiconductor is called p-type semiconductor.
The impurities are called
acceptors as they accept electrons from the semiconductor
Holes are the majority carriers
and the electrons produced by the breaking of bonds are the minority carriers.
Related Topics