Chapter: Basic Electrical and electronics : Semiconductor Devices And Applications
Classification of Materials
CLASSIFICATION OF MATERIALS
The materials are classified based on their
conducting property. Energy band theory can be used to explain the
classification of materials.
1 Conductors
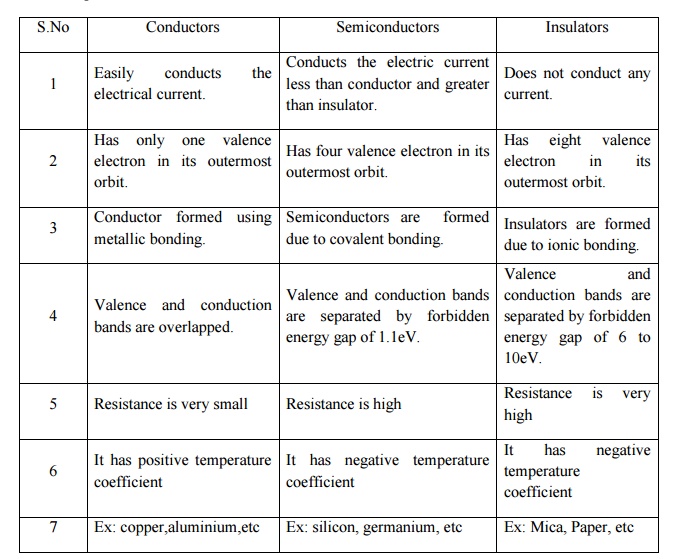
Conductor
is materials that easily conducts or pass the current. There are plenty of free
electrons available for electric conduction. In terms of energy band theory,
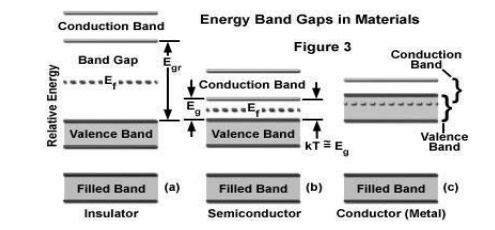
the conductors have overlapping of valence band and conductive band.
Example: Copper,
Aluminum, iron, etc
Properties: 1. It is rigid, non directional and
crystalline in nature.
2. Conductivity
is good.
3. Low
melting and boiling temperatures.
2 Semiconductors
Semiconductor
is a material with partially filled conduction band and valence band. The
current in the semiconductor is due to the movement of electrons and holes. As
the temperature increases the conduction increases.
Example: Silicon,
Germanium, etc.
Properties: 1. It is
rigid, directional and crystalline in nature.
2. Conductivity
can be increased if proper doping material is added.
3. Low
melting and boiling temperatures.
2 Insulators
In the
case of insulators, the valence electrons are very tightly bound to their
parent atom. The valence band and conduction band are separated by a large
forbidden energy gap. The insulators have full valence band and an empty
conduction band.
Example: Paper,
Mica. Sodium chloride, etc.
Properties: 1. It is
rigid, Unidirectional and crystalline in nature.
2.
Conductivity is poor in the solid form.
3. High
melting and boiling temperatures.
Energy band structure
Comparison of
Conductors,Semiconductors and Insulators
Related Topics