Chapter: Environmental Biotechnology: Phytotechnology and Photosynthesis
Carbon sequestration
Carbon sequestration
Their use as a carbon sink is a simpler process, only requiring the
algae them-selves. However, even as a functional algal monoculture, just as
with the joint algal/bacterial bioprocessing for effluents, without external
intervention to limit the standing burden of biomass within the bioreactor,
reduced efficiency and, ultimately, system collapse is inevitable.
In nature, huge amounts of
many elements are held in global reservoirs, reg-ulated by biogeochemical
cycles, driven by various interrelated biological and chemical systems. For
carbon, a considerable mass is held in organic and inor-ganic oceanic stores,
with the seas themselves being dynamic and important component parts of the
planetary carbon cycle. Marine phytoplankton utilise car-bon dissolved in the
water during photosynthesis, incorporating it into biomass and simultaneously
increasing the inflow gradient from the atmosphere. When these organisms die,
they sink, locking up this transient carbon and taking it out of the upper
oceanic ‘fast’ cycle into the ‘slow’ cycle, which is bounded by long-term
activities within the deep ocean sediments. In this respect, the system may be
likened to a biological sequestration pump, effectively removing atmo-spheric
CO2 from circulation within the biosphere on an extended basis. The number,
mass and extent of phytoplankton throughout the world’s seas thus pro-vide a
carbon-buffering capacity on a truly enormous scale, the full size of which has
only really become apparent within the last 10 – 15 years, with the benefit of
satellite observation.
In the century since its
effectiveness as a means of trapping heat in the atmo-sphere was first
demonstrated by the Swedish scientist, Svante Arrhenius, the importance of
reducing the global carbon dioxide emissions has come to be widely appreciated.
The increasing quantities of coal, oil and gas that are burnt for energy has
led to CO2 emissions worldwide becoming more than 10 times higher than they
were in 1900 and there is over 30% more CO2 in the air, cur-rently around 370
parts per million (ppm), than before the Industrial Revolution. Carbon dioxide
is responsible for over 80% of global warming and according to analysis of
samples of the Antarctic ice, the world today has higher levels of greenhouse
gases than at any time in the past 400 000 years. The UN Intergov-ernmental
Panel on Climate Change has warned that immediate action is required to prevent
further atmospheric increases above today’s level. In the absence of swift and
effective measures to control the situation, by 2100 they predict that carbon
dioxide concentrations will rise to 550 ppm on the basis of their lowest
emission model, or over 830 ppm in the highest.
In 1990, over 95% of the
western industrialised nations’ emissions resulted from burning fossil fuels
for energy, with the 25% of the world’s population who live in these countries
consuming nearly 80% of the energy produced globally. Unsurprisingly, energy
industries account for the greatest share (36%) of carbon dioxide emissions, a
large 1000 Megawatt coal-fired power station releases some-thing in the region
of 5 1/2 million tonnes of CO2 annually.
Clearly, the current focus on reducing fossil fuel usage, and on minimising the
emissions of car-bon dioxide to atmosphere, is important. In one sense, the
most straightforward solution to the problem is simply to stop using fossil
fuels altogether. However, this is a rather simplistic view and just too impractical.
While great advances have been made in the field of renewable energy, a
wholesale substitution for gas, coal and oil is not possible at this time if
energy usage is to continue at an unabated rate. The potential role of existing
nonfossil fuel technology to bridge the gap between the current status quo and
a future time, when renewables meet the needs of mankind, is a vital one.
However, it is ridiculous to pretend that this can be achieved overnight,
unless the ‘global village’ really is to consist of just so many mud huts.
In many respects, here is
another case where, if we cannot do the most good, then perhaps we must settle
for doing the least harm and the application of phytotechnology stands as one
very promising means by which to achieve this goal. The natural contribution of
algal photosynthesis to carbon sequestration has already been alluded to and
the use of these organisms in an engineered system to reduce CO2
releases, simply capitalises on this same inherent potential in an unaltered
way.
There have been attempts to
commercialise the benefits of algae as carbon sinks. In the early 1990s, two
prototype systems were developed in the UK, aimed at the reduction of CO2
emissions from various forms of existing combus-tion processes. The BioCoil was
a particularly interesting integrated approach, removing carbon dioxide from
generator emissions and deriving an alternative fuel source in the process. The
process centred on the use of unicellular algal species in a narrow,
water-containing, spiral tube made of translucent polymer, through which the
exhaust gases from the generator was passed. The carbon dioxide rich waters
provided the resident algal with optimised conditions for photosynthesis which
were further enhanced by the use of additional artificial light. The algal
biomass recovered from the BioCoil reactor was dried, and being unicellular,
the effective individual particle size tended to the dimensions of diesel
injection droplets, which, coupled with an energy value roughly equiva-lent to
medium grade bituminous coal at 25 MJ/kg, makes it ideal for use in a suitable
engine without further modification. Despite early interest, the system does
not appear to have been commercially adopted or developed further.
Around the same time, another
method was also suggested by one of the authors. In this case, it was his
intent specifically to deal with the carbon diox-ide produced when biogas, made
either at landfill sites or anaerobic digestion plants, was flared or used for
electricity generation. Termed the algal cultiva-tion system and carbon sink
(ACSACS), it used filamentous algae, growing as attached biofilter elements on
a polymeric lattice support. CO2 rich exhaust gas
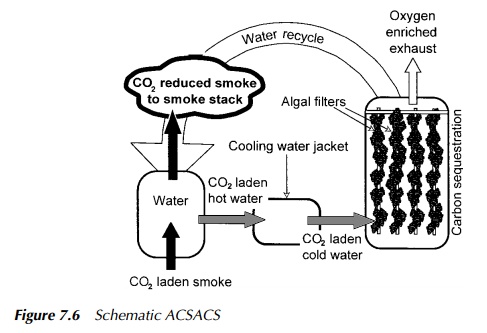
was passed into the bottom of a bioreactor vessel, containing the
plastic filter elements in water, and allowed to bubble up to the surface
through the algal strands as shown in Figure 7.6.
Again, this approach to
carbon sequestration was based on enhanced intra-reactor photosynthesis, the
excess algal biomass being harvested to ensure the ongoing viability of the
system, with the intention of linking it into a compost-ing operation to
achieve the long-term carbon lock-up desired. The ACSACS though performing well
at both bench and small pilot scale, never attained indus-trial adoption though
remaining an interesting possible adjunct to the increasing demand for methane
flaring or utilisation at landfills.
A similar idea emerged again
recently, with a system being developed by Ohio University, which, in a perfect
example of selecting an organism from an extreme environment to match the
demands of a particular manmade situation, utilises thermophilic algae from hot
springs in Yellowstone National Park. In this process, which has received a $1
million grant from the US Department of Energy, smoke from power stations is
diverted through water to permit some of the CO2 to be absorbed and
the hot, carbonated water produced then flows through an algal filter formed on
vertical nylon screens.
This design, which is
essentially similar to the earlier ACSACS, enables the largest possible algal
population per unit volume to be packed into the filter unit, though like the
previously described HRAP, light is a limiting factor, since direct sunlight
will only penetrate through a few feet of such an arrangement. However, it is
claimed that these carbon biofilters could remove up to 20% of the carbon
dioxide, which would, of course, otherwise be released to atmosphere. This makes
solving the problem something of a priority. One solution involves the use of a
centralised light collector, connected to a series of fibre-optic cables linked
to diffusers within the vessels to provide adequate illumination within the
filters. An alternative approach has been put forward, using large artificial
lakes, but this would require a much larger land bank to produce the same
effect, since they would have to be very shallow by comparison. It has also
been suggested that cooling the carbonated water first, a feature of both the
BioCoil and ACSACS, would allow normal mesophilic algae to be used, which take
up CO2 more efficiently. Whether this technique will prove any more
successful in gaining industrial or commercial acceptance than either of the
earlier British systems remains to be seen.
Related Topics